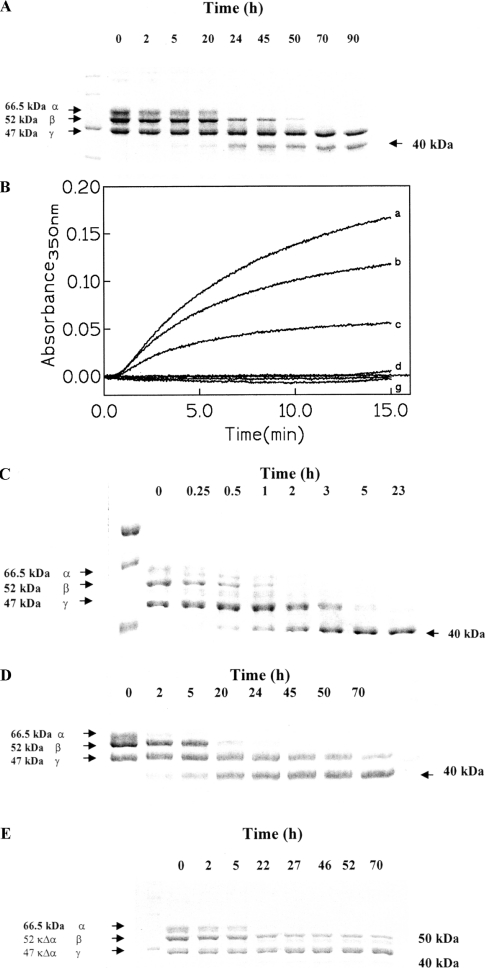
Figure 1. Kinetic proteolytic processing of bovine fibrinogen.
(A) SDS/PAGE electrophoresis of the processing of bovine fibrinogen by whole MMP-2 as a function of time (as indicated) at 37 °C and pH 7.1. α- (66.5 kDa), β- (52 kDa) and γ- (47 kDa) chains are indicated as well as the 40 kDa fragment. (B) Clotting induced by human thrombin on bovine fibrinogen (curves a–c) and on bovine fibrinogen after degradation by whole MMP-2 (curves d–g). Fibrinogen was incubated at 37 °C in 50 mM Tris/HCl buffer (pH 8.2) and the clotting was followed at 350 nm after addition of 1.2 units of human thrombin. Curves a–c: 0.24, 0.16 and 0.08 mg/ml of bovine fibrinogen. Curves d–g: 0.08, 0.16, 0.24 and 0.32 mg/ml of fibrinogen degraded by whole MMP-2. (C) SDS/PAGE electrophoresis of the processing of bovine fibrinogen by plasmin as a function of time (as indicated) at 37 °C and pH 7.1. α- (66.5 kDa), β- (52 kDa) and γ- (47 kDa) chains are indicated as well as the 40 kDa fragment. (D) SDS/PAGE electrophoresis of the processing of peroxynitritre-treated bovine fibrinogen by MMP-2 as a function of time (as indicated) at 37 °C and pH 7.1. α- (66.5 kDa), β- (52 kDa) and γ- (47 kDa) chains are indicated as well as the 40 kDa fragment. (E) SDS/PAGE electrophoresis of the processing of bovine fibrinogen by the catalytic domain of MMP-2 as a function of time (as indicated) at 37 °C and pH 7.1. α- (66.5 kDa), β- (52 kDa) and γ- (47 kDa) chains are indicated as well as the 50 kDa and the 40 kDa fragments. For further details, see text.