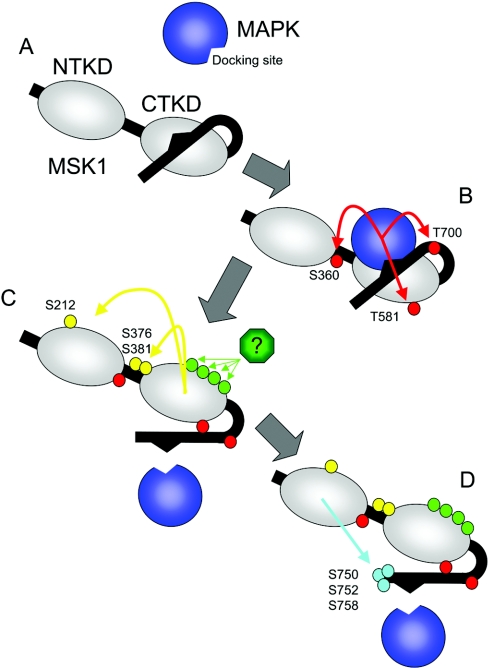
Figure 7. Activation model for MSK1.
MSK1 consists of two kinase domains, joined by a short linker region, and a C-terminal extension containing a MAPK kinase docking domain after the second kinase domain. In the inactive state (A), MSK1 is unphosphorylated and the C-terminus of the protein may fold back and act as an inhibitor of the C-terminal kinase domain, as has been described for the related kinase MAPKAPK2. Active ERK1/2 or p38 MAPK is able to bind to the docking motif in the C-terminal region of MSK1, resulting in the phosphorylation of three sites, Ser360, Thr581 and Thr700 in MSK1 (red circles; B). Thr581 is in the activation loop of the C-terminal kinase domain and its phosphorylation may be required to stabilize the active confirmation of the activation loop. Thr700 is proposed to lie in a hinge region between the C-terminal kinase domain and the inhibitory C-terminal sequence and phosphorylation of this residue would promote the dissociation of the inhibitory sequence from the kinase domain. MSK1 is additionally phosphorylated on a further four sites by an unidentified kinase (green circles), and the function of these phosphorylations is unclear. The activated C-terminal kinase domain then activates the N-terminal kinase domain via the phosphorylation of three sites, Ser212, Ser376 and Ser381 (yellow circles; C). Once activated, the N-terminal kinase domain is able to phosphorylate substrates, and additionally phosphorylates three sites, Ser750, Ser752 and Ser758 (blue circles; D) at the C-terminus of MSK1.