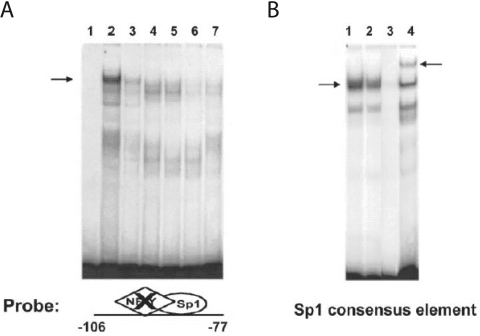
Figure 4. Dietary PUFAs impair Sp1 DNA binding activity.
Hepatic nuclear protein extracts (5 μg per reaction) were prepared from rats fed an FF diet or the FO diet. (A) EMSAs were conducted using the Fasn promoter region of −106 to −77 bp in which the NF-Y site was mutated (−106/−77NF−Ymt) (see Table 1). Lane 1 is free probe. Lanes 2 and 3 show Sp1 binding activity for extracts from rats fed the FF or the FO diet. Specificity of Sp1 binding to the −106/−77NF−Ymt fragment (A) was determined using nuclear proteins from rats fed the FF diet incubated with a 100-fold molar excess of unlabelled −106/−77NF−Ymt, (lane 4), the Sp1 consensus sequence (lane 5), the wild-type −106 to −77 bp Fasn sequence (lane 6) or the anti-Sp1 antibody (lane 7). (B) EMSAs were conducted using an oligonucleotide containing a consensus Sp1 binding sequence (see Table 1) and nuclear protein extracts from rats fed the FF diet (lane 1) or the FO diet (lane 2). Specificity of Sp1 binding to the consensus sequence was determined using nuclear proteins from rats fed the FF diet incubated with a 100-fold molar excess of unlabelled consensus Sp1 site (lane 3) or the anti-Sp1 antibody (lane 4). The left-hand arrows designate the Sp1 binding complex, and the right-hand arrow designates the super-shift resulting from treatment with anti-Sp1 antibody. Reduced Sp1 binding by dietary FO was demonstrated in seven rats with a mean decrease in binding activity of 52±4% (P<0.05) for the −106/−77NF−Ymt and consensus Sp1 sequences.