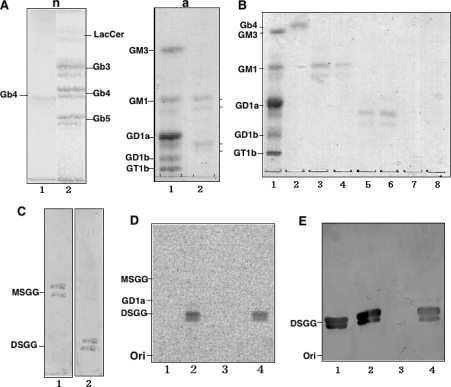
Figure 2. Preparation of globo-series glycosphingolipids from ACHN cells and determination of sialyltransferase activity of hST6GalNAc III/V/VI toward MSGG.
(A) Extraction of neutral (n) and acidic (a) glycolipids from ACHN cells. n, Orcinol-sulfuric acid staining of the neutral fraction. Lane 1, Gb4; lane 2, neutral glycolipid fraction. a, Resorcinol staining of the acidic fraction. Lane 1, bovine brain gangliosides as standards; lane 2, acidic fraction. (B) Purification of MSGG and DSGG. Acidic glycolipids were eluted by step-wise elution with sodium acetate in a solvent system of chloroform/methanol/sodium acetate (30:60:8, by vol., lanes 3–10). Lane 1, ganglioside marker; lane 2, Gb4; lane 3, 0.2 M sodium acetate fraction; lane 4, 0.25 M; lane 5, 0.3 M; lane 6, 0.45 M; lane 7, 0.6 M; and lane 8, 0.8 M. (C) TLC-immunostaining. Lane 1, MSGG (eluted by 0.2 and 0.25 M sodium acetate) was immunostained with mAb RM1. Lane 2, DSGG (eluted by 0.3 and 0.45 M sodium acetate) was immunostained with mAb 5F3. (D) The sialyltransferase assay was performed using the membrane fraction with MSGG as an acceptor substrate as described in the Materials and methods section, and the products were analysed by TLC and autofluorography. Lane 1, products with membrane fractions from L cells transfected with vector control; lane 2, hST6GalNAcIII; lane 3, hST6GalNAc V; lane 4, hST6GalNAc VI. (E) TLC-immunostaining of the enzyme products was performed with mAb 5F3. Lane 1, DSGG (2.5 μg); lanes 2–4, are essentially the same as in (D) except that bands were detected with mAb 5F3.