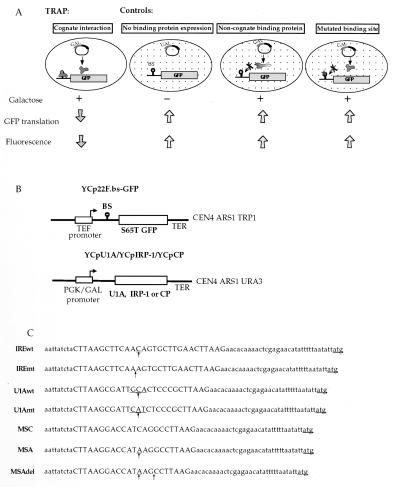
Figure 1.
Schematic representation of TRAP. (A) The principle of TRAP. RS453 cells are transformed with the GFP indicator plasmid that carries within the GFP 5′UTR the binding site of the protein under study and the plasmid for expression of the RNA-binding protein (or a cDNA library) under the control of a galactose-inducible promoter. TRAP: In galactose medium, expression of the RNA-binding protein is induced. The RNA–protein interaction at the 5′ end of the GFP mRNA represses its translation and the fluorescence of the cell is diminished (cognate interaction). Controls: GFP mRNA translation yields high fluorescence levels when expression of the RNA-binding protein is repressed in glucose medium (no binding protein expression), or when the expressed protein cannot interact with the GFP mRNA (noncognate binding protein or mutated binding site). (B) Description of the plasmids used. The GFP reporter plasmids YCp22F.BS-GFP contain the GFP S65T mutant ORF. The IRE and U1 snRNA loop 2 wild type and mutated, as well as the MSC, MSA, and MSAdel binding sites (BS) are cloned into the AflII site. The binding sites are located 9 nucleotides downstream from the transcription start site and 32 nucleotides upstream from the GFP translation initiation codon. The plasmids harbor a TRP1 selection marker. YCpU1A, YCpIRP-1, and YCpCP are used for the galactose-inducible expression of U1A, IRP-1, and MS2 coat protein (CP), respectively. They contain a URA3 selection marker. (C) Sequences and nomenclature of the GFP reporter plasmids. Binding site insertions are represented by capital letters. Differences between wild-type and mutated binding sites are indicated by arrows.