Abstract
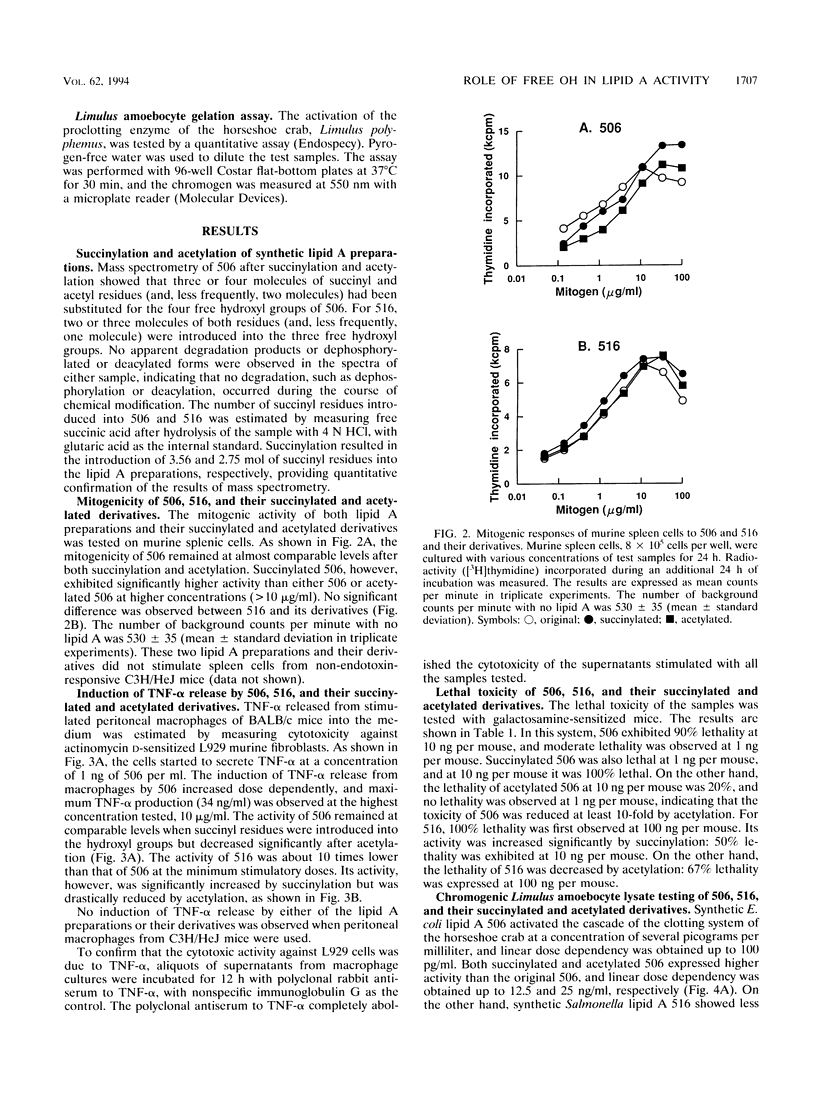
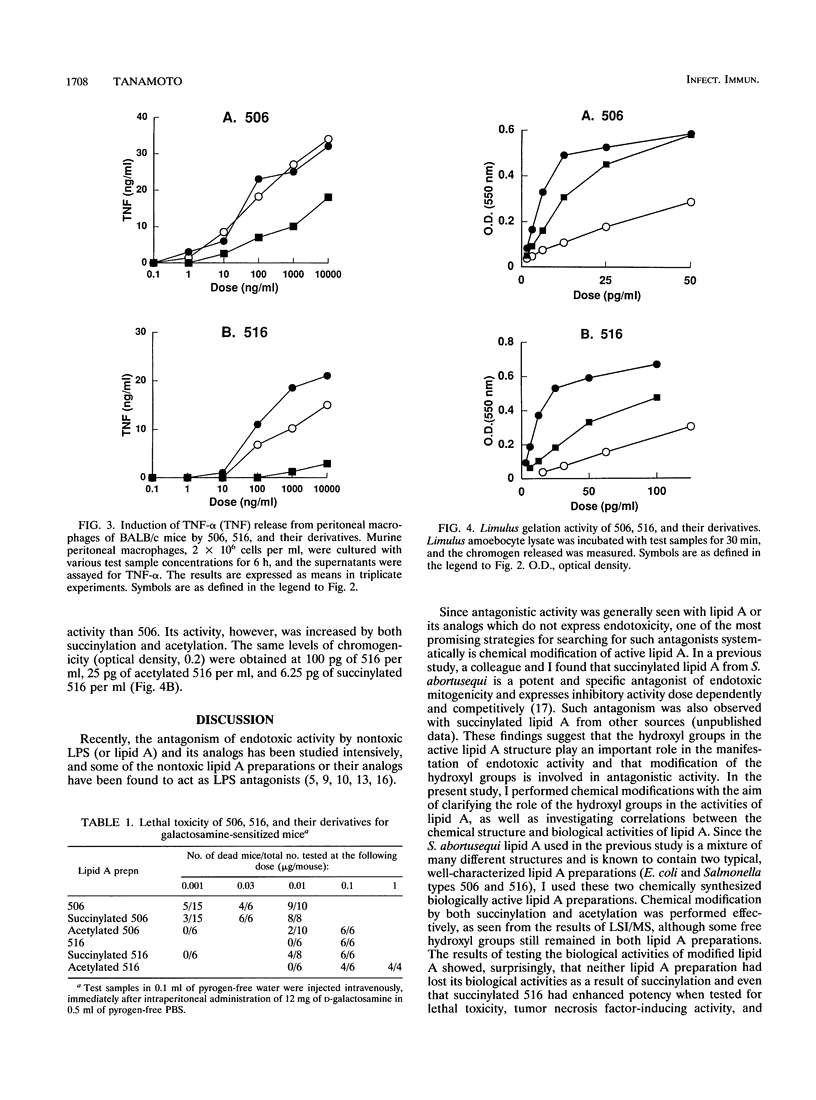
Previous studies demonstrated that lipid A from Salmonella abortusequi loses its B-cell mitogenicity for murine spleen cells as a result of the introduction of succinyl residues on hydroxyl groups and that the inactivated lipid A specifically antagonizes the mitogenicity of endotoxin. Hypothesizing that the hydroxyl groups are essential both for its biological activity and for producing nontoxic preparations having antagonistic activity, I tested the role of the hydroxyl groups in its activities by using well-characterized biologically active lipid A preparations synthesized chemically (Escherichia coli and Salmonella types 506 and 516, respectively) by the introduction of either succinyl or acetyl residues at the hydroxyl groups of each of these lipid A preparations. However, the biological activities of neither lipid A preparation were reduced at all after succinylation; in fact, succinylated 516 became much more potent than the original molecule with respect to most activities tested, i.e., lethal toxicity, Limulus gelation activity, and the induction of tumor necrosis factor release. On the other hand, when the hydroxyl groups were replaced with acetyl residues, the lethality and tumor necrosis factor-inducing activity of both lipid A preparations were decreased, whereas their Limulus gelation activity was increased. Mitogenicity was not affected much by the chemical modifications of either lipid A preparation. These findings indicate that although the residues introduced into the free hydroxyl groups of lipid A modulate its activities, the hydroxyl groups in lipid A need not exist in free form.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fong Y. M., Marano M. A., Moldawer L. L., Wei H., Calvano S. E., Kenney J. S., Allison A. C., Cerami A., Shires G. T., Lowry S. F. The acute splanchnic and peripheral tissue metabolic response to endotoxin in humans. J Clin Invest. 1990 Jun;85(6):1896–1904. doi: 10.1172/JCI114651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanos C., Freudenberg M. A., Reutter W. Galactosamine-induced sensitization to the lethal effects of endotoxin. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5939–5943. doi: 10.1073/pnas.76.11.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanos C., Lehmann V., Lüderitz O., Rietschel E. T., Westphal O., Brade H., Brade L., Freudenberg M. A., Hansen-Hagge T., Lüderitz T. Endotoxic properties of chemically synthesized lipid A part structures. Comparison of synthetic lipid A precursor and synthetic analogues with biosynthetic lipid A precursor and free lipid A. Eur J Biochem. 1984 Apr 16;140(2):221–227. doi: 10.1111/j.1432-1033.1984.tb08090.x. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Rietschel E. T., Westphal O., Brade H., Brade L., Freudenberg M., Schade U., Imoto M., Yoshimura H. Synthetic and natural Escherichia coli free lipid A express identical endotoxic activities. Eur J Biochem. 1985 Apr 1;148(1):1–5. doi: 10.1111/j.1432-1033.1985.tb08798.x. [DOI] [PubMed] [Google Scholar]
- Golenbock D. T., Hampton R. Y., Qureshi N., Takayama K., Raetz C. R. Lipid A-like molecules that antagonize the effects of endotoxins on human monocytes. J Biol Chem. 1991 Oct 15;266(29):19490–19498. [PubMed] [Google Scholar]
- Homma J. Y., Matsuura M., Kanegasaki S., Kawakubo Y., Kojima Y., Shibukawa N., Kumazawa Y., Yamamoto A., Tanamoto K., Yasuda T. Structural requirements of lipid A responsible for the functions: a study with chemically synthesized lipid A and its analogues. J Biochem. 1985 Aug;98(2):395–406. doi: 10.1093/oxfordjournals.jbchem.a135294. [DOI] [PubMed] [Google Scholar]
- Kanegasaki S., Kojima Y., Matsuura M., Homma J. Y., Yamamoto A., Kumazawa Y., Tanamoto K., Yasuda T., Tsumita T., Imoto M. Biological activities of analogues of lipid A based chemically on the revised structural model. Comparison of mediator-inducing, immunomodulating and endotoxic activities. Eur J Biochem. 1984 Sep 3;143(2):237–242. doi: 10.1111/j.1432-1033.1984.tb08364.x. [DOI] [PubMed] [Google Scholar]
- Kanegasaki S., Tanamoto K., Yasuda T., Homma J. Y., Matsuura M., Nakatsuka M., Kumazawa Y., Yamamoto A., Shiba T., Kusumoto S. Structure-activity relationship of lipid A: comparison of biological activities of natural and synthetic lipid A's with different fatty acid compositions. J Biochem. 1986 Apr;99(4):1203–1210. doi: 10.1093/oxfordjournals.jbchem.a135583. [DOI] [PubMed] [Google Scholar]
- Kirkland T. N., Qureshi N., Takayama K. Diphosphoryl lipid A derived from lipopolysaccharide (LPS) of Rhodopseudomonas sphaeroides inhibits activation of 70Z/3 cells by LPS. Infect Immun. 1991 Jan;59(1):131–136. doi: 10.1128/iai.59.1.131-136.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loppnow H., Libby P., Freudenberg M., Krauss J. H., Weckesser J., Mayer H. Cytokine induction by lipopolysaccharide (LPS) corresponds to lethal toxicity and is inhibited by nontoxic Rhodobacter capsulatus LPS. Infect Immun. 1990 Nov;58(11):3743–3750. doi: 10.1128/iai.58.11.3743-3750.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michie H. R., Manogue K. R., Spriggs D. R., Revhaug A., O'Dwyer S., Dinarello C. A., Cerami A., Wolff S. M., Wilmore D. W. Detection of circulating tumor necrosis factor after endotoxin administration. N Engl J Med. 1988 Jun 9;318(23):1481–1486. doi: 10.1056/NEJM198806093182301. [DOI] [PubMed] [Google Scholar]
- Proctor R. A., Will J. A., Burhop K. E., Raetz C. R. Protection of mice against lethal endotoxemia by a lipid A precursor. Infect Immun. 1986 Jun;52(3):905–907. doi: 10.1128/iai.52.3.905-907.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietschel E. T., Galanos C., Tanaka A., Ruschmann E., Lüderitz O., Westphal O. Biological activities of chemically modified endotoxins. Eur J Biochem. 1971 Sep 24;22(2):218–224. doi: 10.1111/j.1432-1033.1971.tb01535.x. [DOI] [PubMed] [Google Scholar]
- Suffredini A. F., Fromm R. E., Parker M. M., Brenner M., Kovacs J. A., Wesley R. A., Parrillo J. E. The cardiovascular response of normal humans to the administration of endotoxin. N Engl J Med. 1989 Aug 3;321(5):280–287. doi: 10.1056/NEJM198908033210503. [DOI] [PubMed] [Google Scholar]
- Tanamoto K., Galanos C., Lüderitz O., Kusumoto S., Shiba T. Mitogenic activities of synthetic lipid A analogs and suppression of mitogenicity of lipid A. Infect Immun. 1984 May;44(2):427–433. doi: 10.1128/iai.44.2.427-433.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanamoto K., Ishibashi N. Succinylated lipid A is a potent and specific inhibitor of endotoxin mitogenicity. J Gen Microbiol. 1992 Dec;138(12):2503–2508. doi: 10.1099/00221287-138-12-2503. [DOI] [PubMed] [Google Scholar]
- Tanamoto K., Schade U., Rietschel E. T. Sensitization of alveolar macrophages to lipopolysaccharide-induced prostaglandin synthesis by exogenous prostaglandins. Biochem Biophys Res Commun. 1989 Nov 30;165(1):526–532. doi: 10.1016/0006-291x(89)91101-7. [DOI] [PubMed] [Google Scholar]
- Tanamoto K., Shade U., Rietschel E. T., Kusumuto S., Shiba T. Endotoxic induction of prostaglandin release from macrophages by nontoxic lipid A analogs synthesized chemically. Infect Immun. 1990 Jan;58(1):217–221. doi: 10.1128/iai.58.1.217-221.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanamoto K., Zähringer U., McKenzie G. R., Galanos C., Rietschel E. T., Lüderitz O., Kusumoto S., Shiba T. Biological activities of synthetic lipid A analogs: pyrogenicity, lethal toxicity, anticomplement activity, and induction of gelation of Limulus amoebocyte lysate. Infect Immun. 1984 May;44(2):421–426. doi: 10.1128/iai.44.2.421-426.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]


