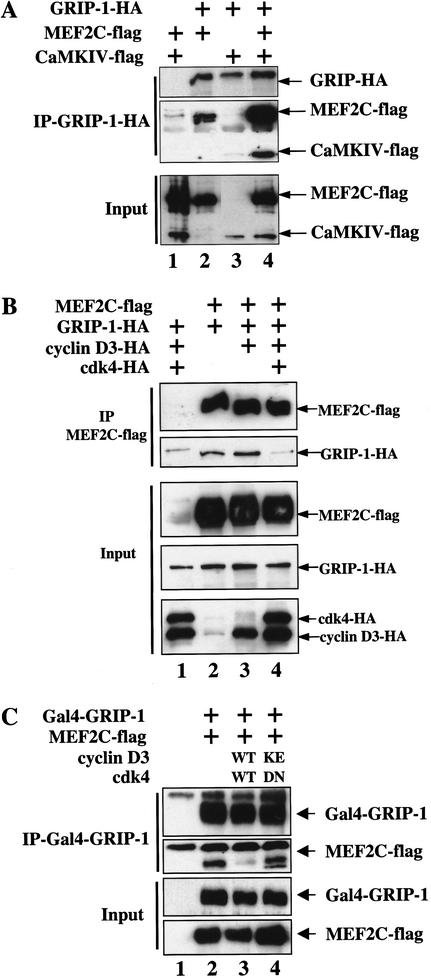
Figure 5.
Kinase-dependent inhibition of GRIP-1–MEF2C association by cyclin D3–cdk4 in vivo. (A) Activated CaMKIV augments the association between MEF2 and GRIP-1. COS cells were cotransfected with plasmids encoding MEF2C–Flag (lanes 1,2,4), GRIP-1–HA (lanes 2–4), and CaMKIV-Flag (lanes 1,3,4). Cells were lysed, and anti-GRIP-1–HA immunoprecipitations were performed. Western blot analysis for the indicated proteins is shown for either the input cell lysate or for material immunoprecipitated by the anti-HA antibody. (B) Cyclin D3–cdk4 disrupts the interaction between MEF2 and GRIP-1. COS cells were transfected with plasmids encoding MEF2C–Flag (lanes 2–4), GRIP-1–HA (lanes 1–4), cyclin D3 (lanes 1,3,4), and cdk4 (lanes 1,4). Cells were lysed and anti-MEF2C–Flag immunoprecipitations were performed. Western blot analysis for the indicated proteins is shown for either the input cell lysate or for material immunoprecipitated by the anti-Flag antibody. (C) Cyclin D3–cdk4 catalytic activity is necessary to disrupt the interaction between MEF2 and GRIP-1. COS cells were transfected with plasmids encoding MEF2C–Flag and Gal4GRIP-1 (lanes 2–4), cyclin D3WT and cdk4WT (lane 3), or cyclin D3KE and cdk4DN (lane 4). Cells were lysed and anti-Gal4GRIP-1 immunoprecipitations were performed. Western blot analysis for the indicated proteins is shown for either the input cell lysate or for material immunoprecipitated by the anti-Gal4 antibody.