Abstract
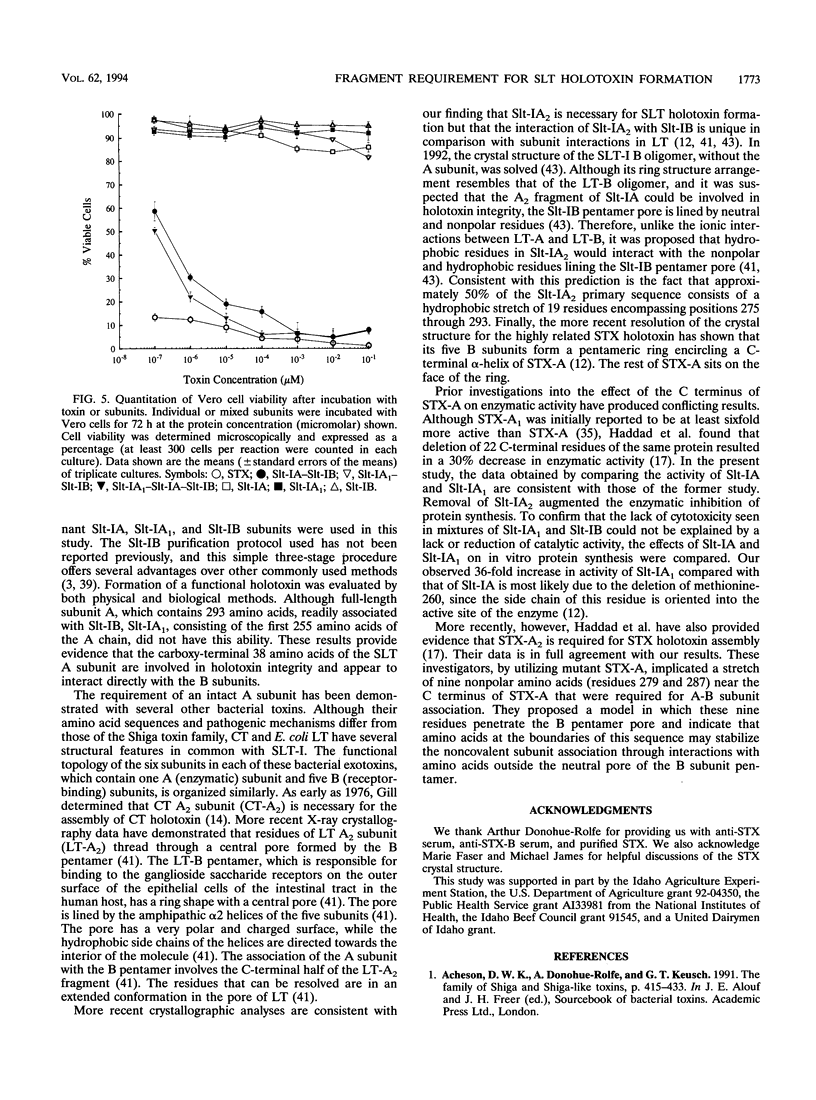
Escherichia coli Shiga-like toxin type I (SLT-I) is a potent cytotoxin consisting of an enzymatically active A subunit and a pentameric B subunit that mediates toxin binding to susceptible eukaryotic cells. Evidence that the carboxy-terminal 38 amino acids of the A subunit are involved in holotoxin 1A:5B association is presented. We compared the ability of purified recombinant SLT-I B subunit (Slt-IB) to combine in vitro with purified recombinant SLT-I A subunit (Slt-IA; full-length subunit A includes amino acids 1 to 293) and its ability to combine with purified recombinant SLT-I A1 subunit (Slt-IA1; truncated subunit A includes amino acids 1 to 255). Each mixture was analyzed for biological and physical evidence of toxin assembly. Although Slt-IA successfully combined with Slt-IB to form a molecular species similar to holotoxin that was detectable by nondenaturing polyacrylamide gel electrophoresis and immunoblotting and yielded a molecule which was cytotoxic to cultured Vero cells, Slt-IA1 did not have this ability. Slt-IA1 was 36-fold more active than Slt-IA in an in vitro protein synthesis inhibition assay. These findings suggest that the Slt-IA2 fragment is crucial for formation of SLT holotoxin and stabilizes the interaction between the A and B subunits.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown J. E., Ussery M. A., Leppla S. H., Rothman S. W. Inhibition of protein synthesis by Shiga toxin: activation of the toxin and inhibition of peptide elongation. FEBS Lett. 1980 Aug 11;117(1):84–88. doi: 10.1016/0014-5793(80)80918-5. [DOI] [PubMed] [Google Scholar]
- Calderwood S. B., Acheson D. W., Goldberg M. B., Boyko S. A., Donohue-Rolfe A. A system for production and rapid purification of large amounts of the Shiga toxin/Shiga-like toxin I B subunit. Infect Immun. 1990 Sep;58(9):2977–2982. doi: 10.1128/iai.58.9.2977-2982.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderwood S. B., Auclair F., Donohue-Rolfe A., Keusch G. T., Mekalanos J. J. Nucleotide sequence of the Shiga-like toxin genes of Escherichia coli. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4364–4368. doi: 10.1073/pnas.84.13.4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanter N., Hall G. A., Bland A. P., Hayle A. J., Parsons K. R. Dysentery in calves caused by an atypical strain of Escherichia coli (S102-9). Vet Microbiol. 1986 Sep;12(3):241–253. doi: 10.1016/0378-1135(86)90053-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Grandis S., Ginsberg J., Toone M., Climie S., Friesen J., Brunton J. Nucleotide sequence and promoter mapping of the Escherichia coli Shiga-like toxin operon of bacteriophage H-19B. J Bacteriol. 1987 Sep;169(9):4313–4319. doi: 10.1128/jb.169.9.4313-4319.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGrandis S., Law H., Brunton J., Gyles C., Lingwood C. A. Globotetraosylceramide is recognized by the pig edema disease toxin. J Biol Chem. 1989 Jul 25;264(21):12520–12525. [PubMed] [Google Scholar]
- Deresiewicz R. L., Austin P. R., Hovde C. J. The role of tyrosine-114 in the enzymatic activity of the Shiga-like toxin I A-chain. Mol Gen Genet. 1993 Nov;241(3-4):467–473. doi: 10.1007/BF00284701. [DOI] [PubMed] [Google Scholar]
- Donohue-Rolfe A., Keusch G. T. Shigella dysenteriae 1 cytotoxin: periplasmic protein releasable by polymyxin B and osmotic shock. Infect Immun. 1983 Jan;39(1):270–274. doi: 10.1128/iai.39.1.270-274.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon V. P., Teerling C., Masri S. A., Gyles C. L. Molecular cloning and nucleotide sequence of another variant of the Escherichia coli Shiga-like toxin II family. J Gen Microbiol. 1990 Jun;136(6):1125–1135. doi: 10.1099/00221287-136-6-1125. [DOI] [PubMed] [Google Scholar]
- Gill D. M. The arrangement of subunits in cholera toxin. Biochemistry. 1976 Mar 23;15(6):1242–1248. doi: 10.1021/bi00651a011. [DOI] [PubMed] [Google Scholar]
- Goldberg I., Mekalanos J. J. Cloning of the Vibrio cholerae recA gene and construction of a Vibrio cholerae recA mutant. J Bacteriol. 1986 Mar;165(3):715–722. doi: 10.1128/jb.165.3.715-722.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin D. E., Gemski P. Release of Shiga toxin from Shigella dysenteriae 1 by polymyxin B. Infect Immun. 1983 Apr;40(1):425–428. doi: 10.1128/iai.40.1.425-428.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad J. E., al-Jaufy A. Y., Jackson M. P. Minimum domain of the Shiga toxin A subunit required for enzymatic activity. J Bacteriol. 1993 Aug;175(16):4970–4978. doi: 10.1128/jb.175.16.4970-4978.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H., Yutsudo T., Hirayama T., Takeda Y. Isolation and some properties of A and B subunits of Vero toxin 2 and in vitro formation of hybrid toxins between subunits of Vero toxin 1 and Vero toxin 2 from Escherichia coli O157:H7. Microb Pathog. 1988 Sep;5(3):189–195. doi: 10.1016/0882-4010(88)90021-6. [DOI] [PubMed] [Google Scholar]
- Jacewicz M., Clausen H., Nudelman E., Donohue-Rolfe A., Keusch G. T. Pathogenesis of shigella diarrhea. XI. Isolation of a shigella toxin-binding glycolipid from rabbit jejunum and HeLa cells and its identification as globotriaosylceramide. J Exp Med. 1986 Jun 1;163(6):1391–1404. doi: 10.1084/jem.163.6.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M. P. Structure-function analyses of Shiga toxin and the Shiga-like toxins. Microb Pathog. 1990 Apr;8(4):235–242. doi: 10.1016/0882-4010(90)90050-z. [DOI] [PubMed] [Google Scholar]
- Keusch G. T., Donohue-Rolfe A., Jacewicz M., Kane A. V. Shiga toxin: production and purification. Methods Enzymol. 1988;165:152-62, 399-401. doi: 10.1016/s0076-6879(88)65025-7. [DOI] [PubMed] [Google Scholar]
- Konowalchuk J., Speirs J. I., Stavric S. Vero response to a cytotoxin of Escherichia coli. Infect Immun. 1977 Dec;18(3):775–779. doi: 10.1128/iai.18.3.775-779.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lindberg A. A., Brown J. E., Strömberg N., Westling-Ryd M., Schultz J. E., Karlsson K. A. Identification of the carbohydrate receptor for Shiga toxin produced by Shigella dysenteriae type 1. J Biol Chem. 1987 Feb 5;262(4):1779–1785. [PubMed] [Google Scholar]
- Locht C., Keith J. M. Pertussis toxin gene: nucleotide sequence and genetic organization. Science. 1986 Jun 6;232(4755):1258–1264. doi: 10.1126/science.3704651. [DOI] [PubMed] [Google Scholar]
- Murzin A. G. OB(oligonucleotide/oligosaccharide binding)-fold: common structural and functional solution for non-homologous sequences. EMBO J. 1993 Mar;12(3):861–867. doi: 10.1002/j.1460-2075.1993.tb05726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicosia A., Perugini M., Franzini C., Casagli M. C., Borri M. G., Antoni G., Almoni M., Neri P., Ratti G., Rappuoli R. Cloning and sequencing of the pertussis toxin genes: operon structure and gene duplication. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4631–4635. doi: 10.1073/pnas.83.13.4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien A. D., Holmes R. K. Shiga and Shiga-like toxins. Microbiol Rev. 1987 Jun;51(2):206–220. doi: 10.1128/mr.51.2.206-220.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsnes S., Reisbig R., Eiklid K. Subunit structure of Shigella cytotoxin. J Biol Chem. 1981 Aug 25;256(16):8732–8738. [PubMed] [Google Scholar]
- Paton A. W., Paton J. C., Goldwater P. N., Heuzenroeder M. W., Manning P. A. Sequence of a variant Shiga-like toxin type-I operon of Escherichia coli O111:H-. Gene. 1993 Jul 15;129(1):87–92. doi: 10.1016/0378-1119(93)90700-d. [DOI] [PubMed] [Google Scholar]
- Reisbig R., Olsnes S., Eiklid K. The cytotoxic activity of Shigella toxin. Evidence for catalytic inactivation of the 60 S ribosomal subunit. J Biol Chem. 1981 Aug 25;256(16):8739–8744. [PubMed] [Google Scholar]
- Ribi H. O., Ludwig D. S., Mercer K. L., Schoolnik G. K., Kornberg R. D. Three-dimensional structure of cholera toxin penetrating a lipid membrane. Science. 1988 Mar 11;239(4845):1272–1276. doi: 10.1126/science.3344432. [DOI] [PubMed] [Google Scholar]
- Sandvig K., Garred O., Holm P. K., van Deurs B. Endocytosis and intracellular transport of protein toxins. Biochem Soc Trans. 1993 Aug;21(3):707–711. doi: 10.1042/bst0210707. [DOI] [PubMed] [Google Scholar]
- Saxena S. K., O'Brien A. D., Ackerman E. J. Shiga toxin, Shiga-like toxin II variant, and ricin are all single-site RNA N-glycosidases of 28 S RNA when microinjected into Xenopus oocytes. J Biol Chem. 1989 Jan 5;264(1):596–601. [PubMed] [Google Scholar]
- Sixma T. K., Aguirre A., Terwisscha van Scheltinga A. C., Wartna E. S., Kalk K. H., Hol W. G. Heat-labile enterotoxin crystal forms with variable A/B5 orientation. Analysis of conformational flexibility. FEBS Lett. 1992 Jun 29;305(2):81–85. doi: 10.1016/0014-5793(92)80869-i. [DOI] [PubMed] [Google Scholar]
- Sixma T. K., Pronk S. E., Kalk K. H., Wartna E. S., van Zanten B. A., Witholt B., Hol W. G. Crystal structure of a cholera toxin-related heat-labile enterotoxin from E. coli. Nature. 1991 May 30;351(6325):371–377. doi: 10.1038/351371a0. [DOI] [PubMed] [Google Scholar]
- Speirs J. I., Stavric S., Konowalchuk J. Assay of Escherichia coli heat-labile enterotoxin with vero cells. Infect Immun. 1977 May;16(2):617–622. doi: 10.1128/iai.16.2.617-622.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein P. E., Boodhoo A., Tyrrell G. J., Brunton J. L., Read R. J. Crystal structure of the cell-binding B oligomer of verotoxin-1 from E. coli. Nature. 1992 Feb 20;355(6362):748–750. doi: 10.1038/355748a0. [DOI] [PubMed] [Google Scholar]
- Tamura M., Nogimori K., Murai S., Yajima M., Ito K., Katada T., Ui M., Ishii S. Subunit structure of islet-activating protein, pertussis toxin, in conformity with the A-B model. Biochemistry. 1982 Oct 26;21(22):5516–5522. doi: 10.1021/bi00265a021. [DOI] [PubMed] [Google Scholar]
- Waddell T., Head S., Petric M., Cohen A., Lingwood C. Globotriosyl ceramide is specifically recognized by the Escherichia coli verocytotoxin 2. Biochem Biophys Res Commun. 1988 Apr 29;152(2):674–679. doi: 10.1016/s0006-291x(88)80091-3. [DOI] [PubMed] [Google Scholar]
- Weinstein D. L., Jackson M. P., Samuel J. E., Holmes R. K., O'Brien A. D. Cloning and sequencing of a Shiga-like toxin type II variant from Escherichia coli strain responsible for edema disease of swine. J Bacteriol. 1988 Sep;170(9):4223–4230. doi: 10.1128/jb.170.9.4223-4230.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yutsudo T., Honda T., Miwatani T., Takeda Y. Physicochemical characterization of A and B subunits of Shiga toxin and reconstitution of holotoxin from isolated subunits. Microbiol Immunol. 1987;31(3):189–197. doi: 10.1111/j.1348-0421.1987.tb03083.x. [DOI] [PubMed] [Google Scholar]