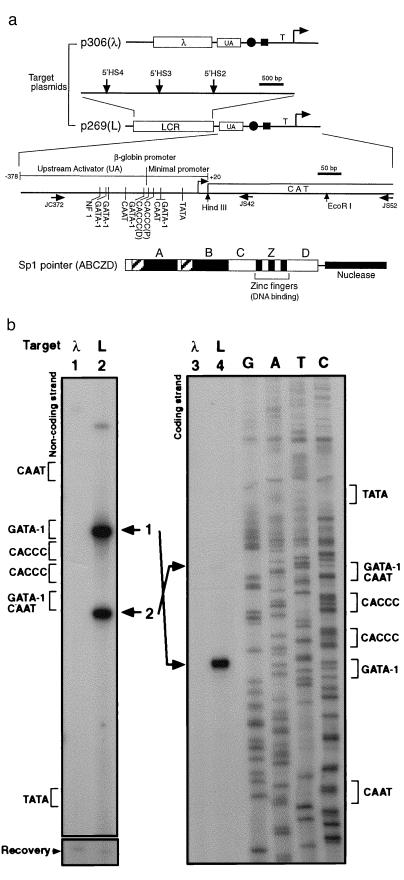
Figure 2.
β-globin LCR recruits Sp1 pointer. (a) Diagram of target plasmids p306 (λ) and p269 (L) is shown at the top. The positions of the hypersensitive sites (β-globin LCR) are indicated with vertical arrows. Also shown are the relative positions of the UA region, the tandem CACCC boxes (solid circle), the overlapping GATA-1/CAAT box (solid rectangle), and the TATA box (T) in the β-globin promoter. The transcription initiation site is indicated by a bent arrow.Transcription factor binding sites in the β-globin upstream activator region and the minimal promoter region (15), the positions of the primers (horizontal arrows) used in this article, and the chloramphenicol acetyltransferase reporter gene (CAT) are shown in the enlarged diagram. The distal and proximal CACCC boxes are indicated as CACCC (D) and CACCC (P), respectively. The structure of the Sp1 pointer ABCZD (16) is shown at the bottom. Domains A and B contain serine/threonine- and glutamine-rich regions, interact with TATA binding protein-associated factors, and are required for transcriptional activation and Sp1 tetramer formation. The zinc fingers (Z) bind DNA in a sequence-specific manner, and domain D is thought to mediate Sp1 tetramer-tetramer interaction (16–18). (b) Recruitment Sp1 pointer ABCZD to target plasmid p306 (λ) (lanes 1 and 3) or p269 (L) (lanes 2 and 4) was detected with PIN*POINT. Primer extensions with radioactively labeled noncoding-strand primer JS42 (Left) and coding-strand primer JC372 (Right) were performed on the recovered DNA. The positions of the promoter elements near the CACCC boxes are indicated for each strand. Bands 1 and 2 (lanes 2 and 4) correspond to the cleavages 5′ and 3′ of the CACCC boxes, respectively. As shown at the bottom (Recovery), the amount of target plasmid in each sample is similar (19).