Figure 4.
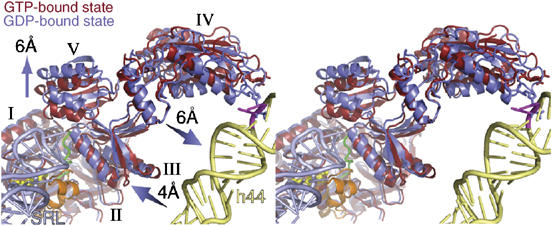
Stereo-view of domain movements in ADPR-eEF2 caused by GTP hydrolysis. The real-space refined, quasi-atomic models of ADPR-eEF2 before (red) and after (blue) GTP hydrolysis are shown together. GTP hydrolysis causes a shift in domains III and V of eEF2 away from domain I. Domain IV and V rotate slightly, as a single rigid body, resulting in a 6 Å shift of the tip of domain IV toward helix 44 in the rRNA of the SSU. The proximity of the SRL (light blue) to the Switch 1 loop (orange) in the GTP-bound state of the quasi-atomic model supports the role of the SRL in catalyzing GTP hydrolysis. GDP is shown in yellow stick representation, the Switch 2 loop is colored green, and the Switch 1 loop in the GTP-bound state is orange. Helix 44 from the 18S rRNA of the SSU is colored light yellow and the SRL from the 25S rRNA of the LSU is colored light blue. The location of two universally conserved adenine bases, A1492 and A1493 (E. coli numbering) in helix 44, that compose part of the ribosomal decoding center, is represented as magenta sticks. This figure was generated with PyMOL (DeLano, 2002).
