Figure 1.
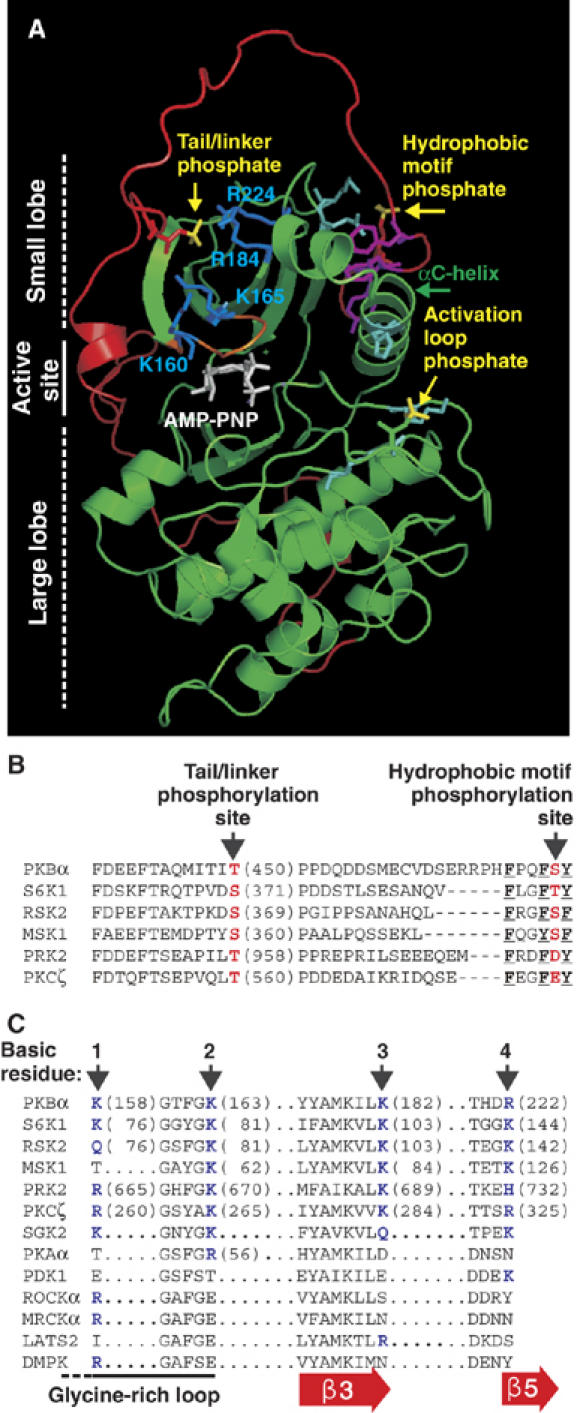
Molecular modelling suggests a widely conserved binding site for the tail/linker phosphoSer/Thr within the catalytic domain of AGC kinases. (A) Model of active PKBβ shown as a ribbon representation with side chains of selected residues. The kinase domain is shown in green and the tail/linker in red. Phosphate groups are shown in yellow. K/R residues predicted to bind the phosphate of phosphoT451 are shown in blue. Other phosphate-binding residues are in cyan. The HM, glycine-rich loop and ATP analogue are shown in magenta, orange and white, respectively. Partial sequence alignment of the tail/linker (B) and predicted binding site for the tail/linker phosphate (C) of AGC kinases. (B, C) Phosphorylation sites/phosphate-mimicking residues are shown in red. The aromatic residues that define the HM are underlined. Basic residues predicted to bind the tail/linker phosphoSer/Thr are shown in blue and labelled 1–4. Sequences are human except S6K1 (rat, p70 isoform) and RSK2 (mouse).
