Figure 5.
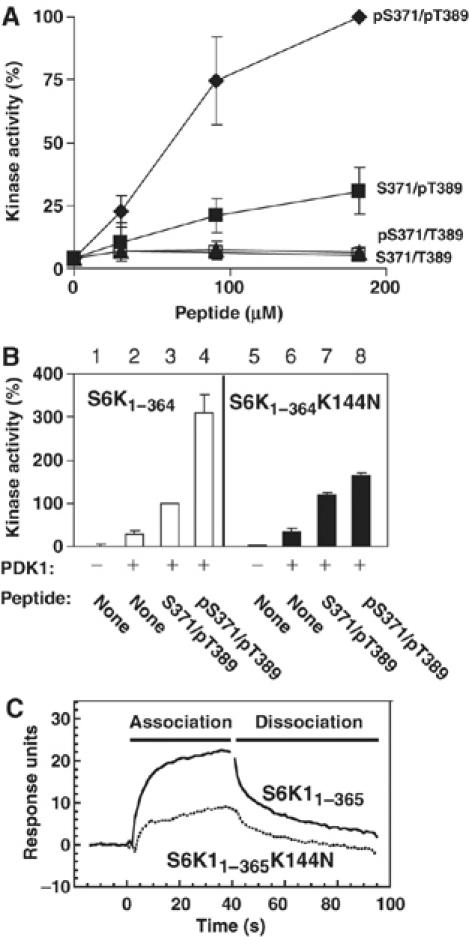
The tail phosphate synergistically enhances the ability of the HM phosphate to activate S6K, dependent on the tail phosphate-binding site. (A) The kinase activity of purified kinase domain of S6K1 (S6K11−364), pre-phosphorylated by PDK1 in the activation loop, was determined in the presence of increasing concentrations of synthetic S6K1 tail peptide (residues 366–395) that was either nonphosphorylated (S371/T389), phosphorylated at Ser371 (pS371/T389) or Thr389 (S371/pT389) or phosphorylated at both sites (pS371/pT389). (B) The kinase activity of S6K11−364 and S6K11−364 K144N, either pre-phosphorylated or not by PDK1, was determined in the absence or presence of 90 μM S371/pT389 or pS371/pT389. (C) pS371/pT389 S6K1 tail peptide was biotinylated and used to coat streptavidin Sensor Chips. The chips were thereafter analysed for binding to purified GST-S6K11−365 or GST-S6K11−365 K144N. (A–C) Experiments were repeated at least three times and activity data (expressed as percent) are means ±s.d.
