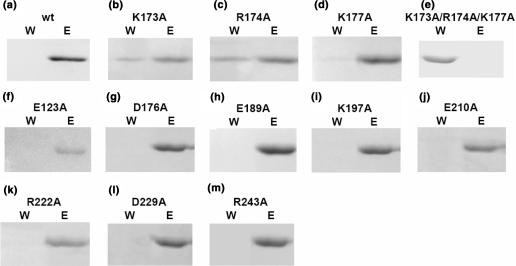
Figure 4.
In vitro pull-down assay on TCS variants suggests that K173, R174 and K177 are involved in binding P2. TCS (a) or its variants (b–m) were loaded to a P2-coupled NHS-Sepharose pre-equilibrated with binding buffer. Bound protein was eluted with 1 M NaCl in 20 mM Tris/HCl buffer pH 8.0. Fractions containing unbound protein collected during washing (W) and bound protein collected during elution (E) were analysed in 15% SDS-PAGE stained with Coomassie blue. As indicated by the presence of TCS in the wash fraction, substitution of alanine at K173, R174 and K177 positions decreases the binding of TCS on P2-coupled column (b–d). Triple-alanine substitutions in these residue positions resulted in a TCS variant (K173A/R174A/K177A) that was unable to bind P2 (e).