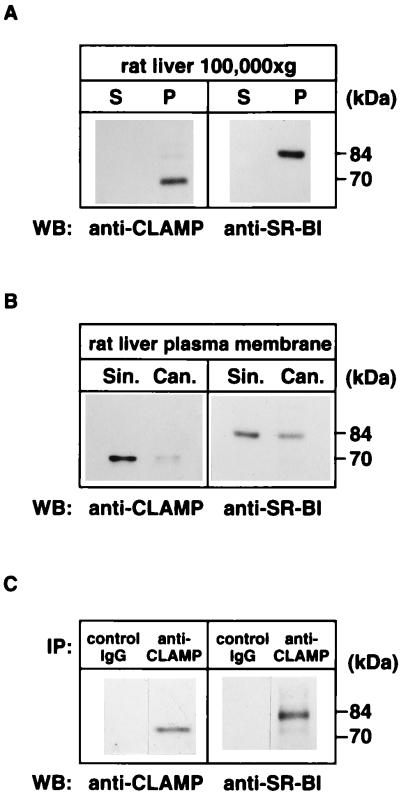
Figure 3.
CLAMP interacts with SR-BI in rat liver sinusoidal membrane. (A) Presence of SR-BI and CLAMP in the membrane fraction. Rat liver homogenate was separated by centrifugation into a soluble fraction (S) and an insoluble membrane pellet fraction (P). Equal fractions (not equal protein) were separated by SDS/PAGE and processed for Western blotting (WB) with an antibody against CLAMP or SR-BI. (B) Localization of SR-BI and CLAMP in the liver sinusoidal and canalicular membranes. Rat liver membranes were separated into a sinusoidal (Sin.) and a canalicular (Can.) membrane fraction as described in Materials and Methods. Equal amounts of protein were separated by SDS/PAGE and processed for Western blotting with an antibody against CLAMP or SR-BI. (C) Coimmunoprecipitation of CLAMP with SR-BI. The rat liver membranes were extracted with Triton X-100, and CLAMP was immunoprecipitated (IP) with either an anti-CLAMP antibody or a control IgG. The immunoprecipitates were then analyzed by Western blotting with an antibody against CLAMP or SR-BI.