Abstract
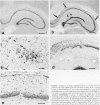
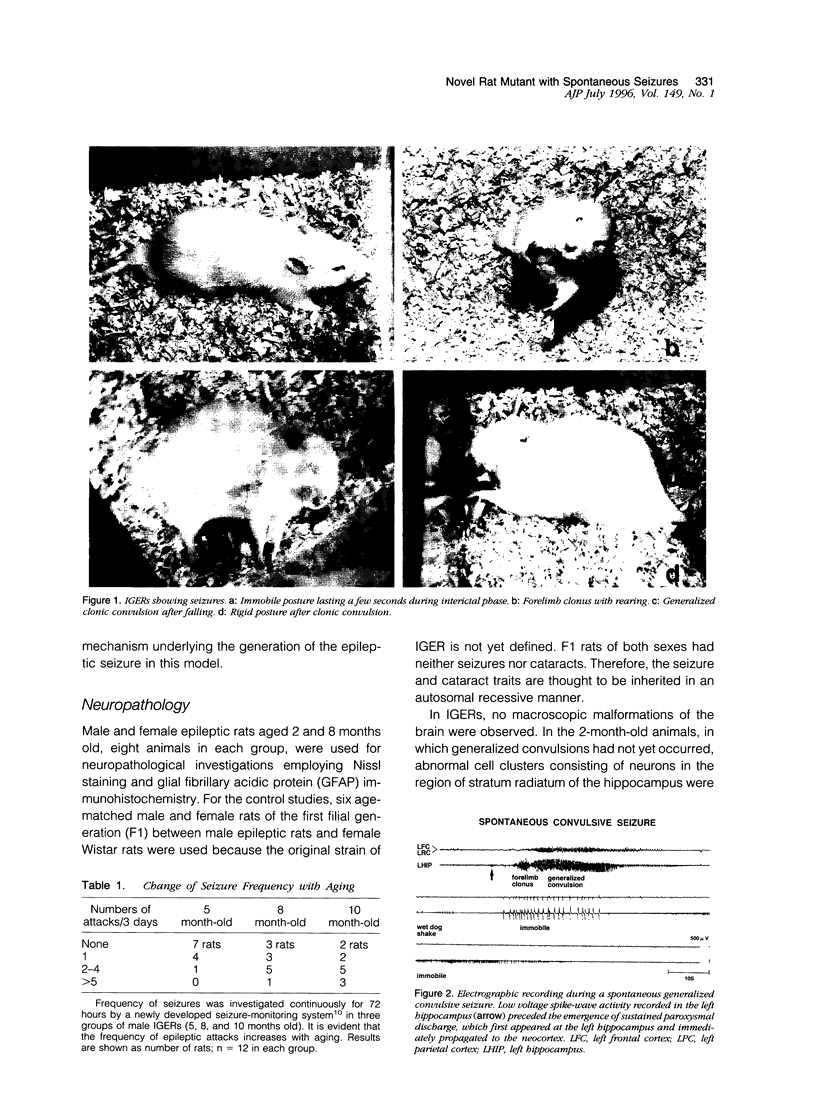
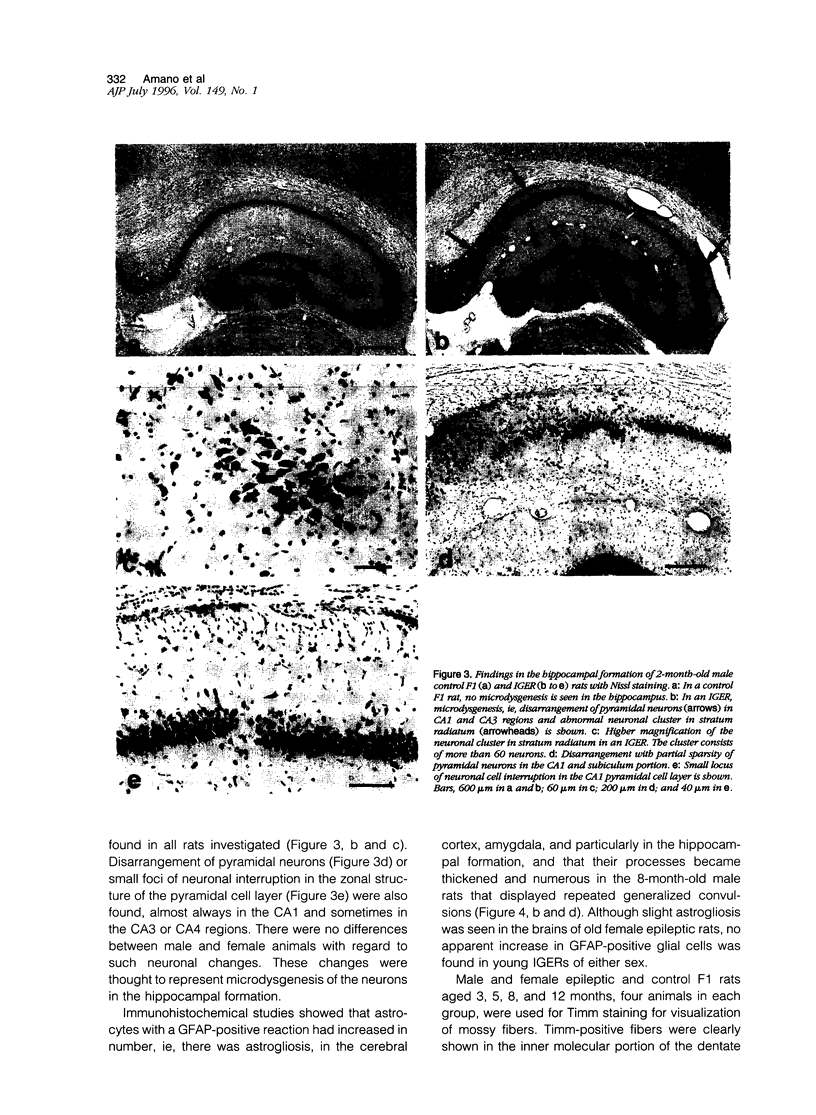
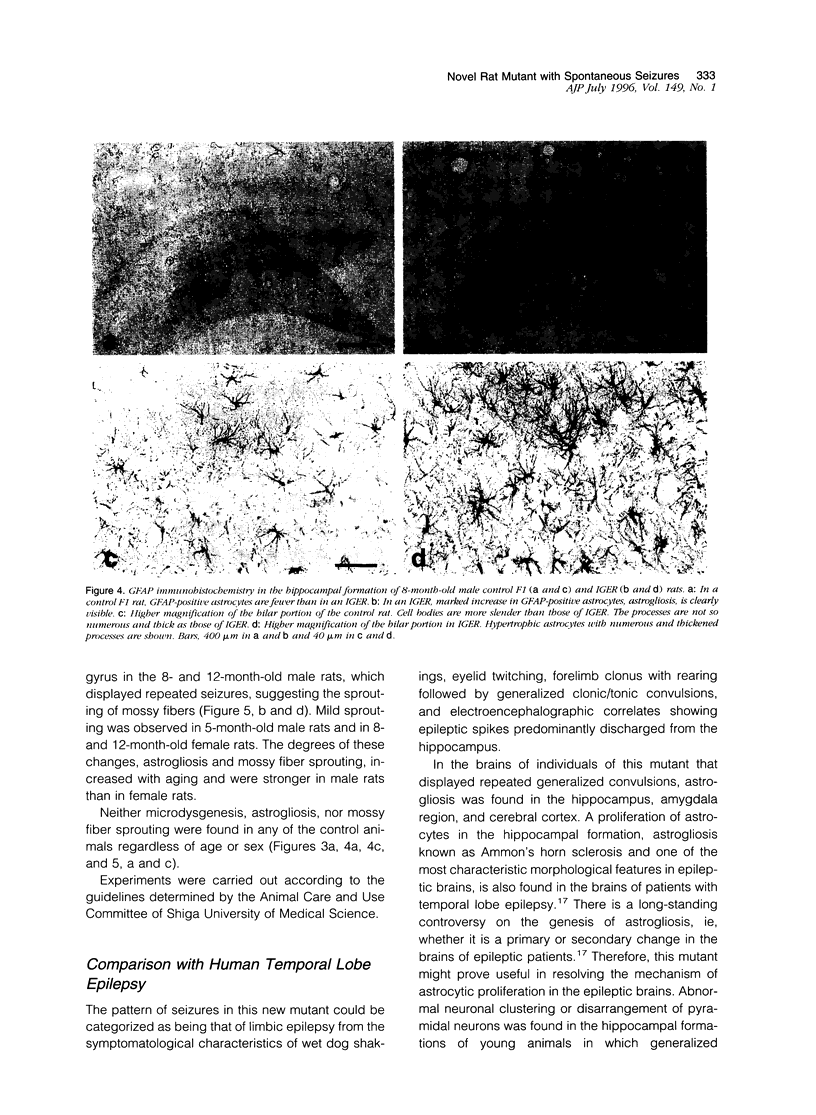
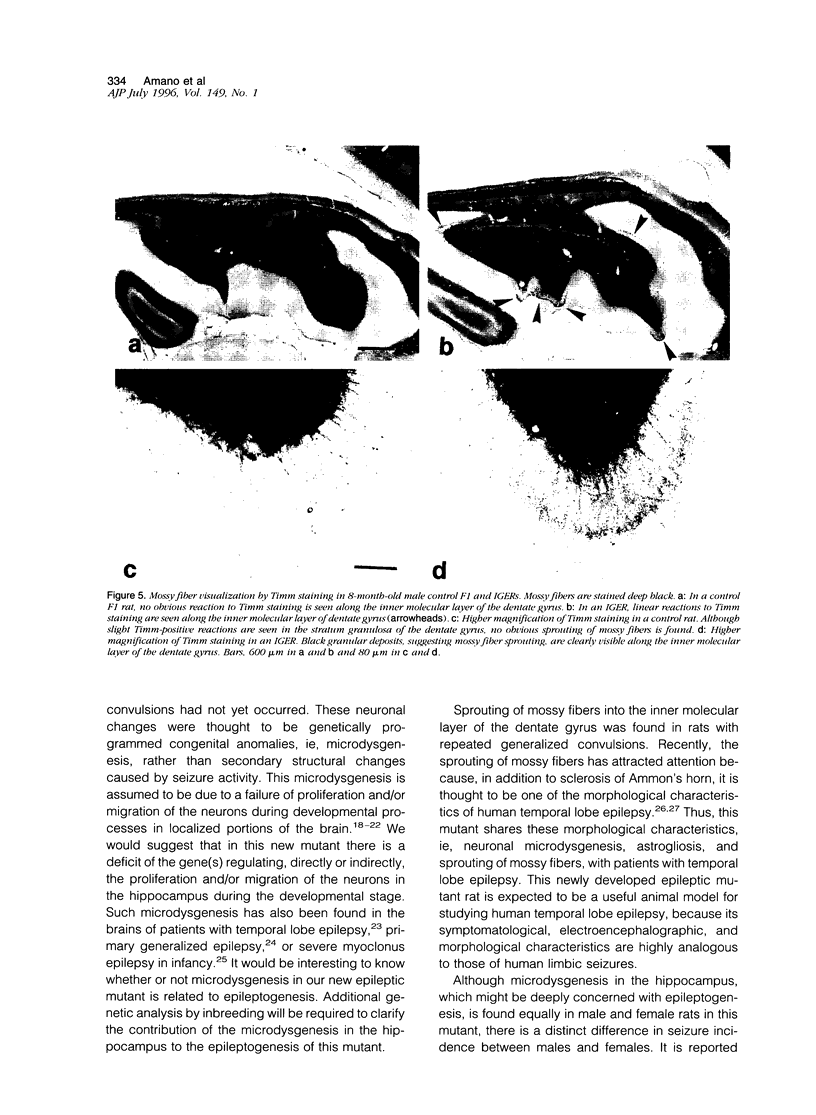
A new epileptic rat mutant with spontaneous seizures was developed by successive mating and selection from an inherited cataract rat. The procedures for developing the mutant and the symptomatology, electroencephalographic correlates, and neuropathology of the mutant are reported. It is possible that this rat stain will provide a useful animal model for human temporal lobe epilepsy. The seizures of the rat usually begin with face and head myoclonus, followed by rearing, and generalized clonic and tonic convulsions, all of which are symptomatologically the same as limbic seizures. Electrographic recording during generalized convulsive seizures demonstrated that sustained spike discharges emerged at the hippocampus and then propagated to the neocortex. Seizures occurred spontaneously without any artificial stimuli. Furthermore, external stimuli such as auditory, flashing light, or vestibular stimulations could not elicit epileptic attacks. Almost all of the male animals had generalized convulsions, mostly from 5 months after birth, and the frequency of the seizures increased with aging. Generalized convulsions developed in approximately 20% of the female rats. Microdysgenesis, such as abnormal neuronal clustering, neuronal disarrangement, or interruption of pyramidal neurons in the hippocampal formation, was found in the young rats that had not yet had generalized seizures. This microdysgenesis, which is though to be genetically programmed, was very interesting from the aspect of the relationship between structural abnormalities and epileptogenesis in this mutant. In addition to microdysgenesis, there was sprouting of mossy fibers into the inner molecular layer of the dentate gyrus in those adult rats that had repeated generalized convulsions. An increase of glial-fibrillary-acidic-protein-positive astrocytes with thickened and numerous processes, ie, astrogliosis, was also found in the cerebral cortex, amygdala region, and hippocampus of these adult animals. Judging from the characteristics of the symptomatology, electroencephalographic correlates, and neuropathology, this epileptic mutant can be expected to be a useful animal model for studying human temporal lobe epilepsy.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amano S., Ihara N., Uemura S., Yokoyama M., Ikeda M., Hazama F. Neuropathological study on a newly developed epileptic rat mutant with limbic-like seizures. Psychiatry Clin Neurosci. 1995 Jun;49(3):S284–S286. doi: 10.1111/j.1440-1819.1995.tb02209.x. [DOI] [PubMed] [Google Scholar]
- Barth P. G. Disorders of neuronal migration. Can J Neurol Sci. 1987 Feb;14(1):1–16. doi: 10.1017/s031716710002610x. [DOI] [PubMed] [Google Scholar]
- Caviness V. S., Jr, Yorke C. H., Jr Interhemispheric neocortical connections of the corpus callosum in the reeler mutant mouse: a study based on anterograde and retrograde methods. J Comp Neurol. 1976 Dec 15;170(4):449–459. doi: 10.1002/cne.901700405. [DOI] [PubMed] [Google Scholar]
- Greenberg D. A., Delgado-Escueta A. V., Widelitz H., Sparkes R. S., Treiman L., Maldonado H. M., Park M. S., Terasaki P. I. Juvenile myoclonic epilepsy (JME) may be linked to the BF and HLA loci on human chromosome 6. Am J Med Genet. 1988 Sep;31(1):185–192. doi: 10.1002/ajmg.1320310125. [DOI] [PubMed] [Google Scholar]
- Hardiman O., Burke T., Phillips J., Murphy S., O'Moore B., Staunton H., Farrell M. A. Microdysgenesis in resected temporal neocortex: incidence and clinical significance in focal epilepsy. Neurology. 1988 Jul;38(7):1041–1047. doi: 10.1212/wnl.38.7.1041. [DOI] [PubMed] [Google Scholar]
- Hom A. C., Buterbaugh G. G. Estrogen alters the acquisition of seizures kindled by repeated amygdala stimulation or pentylenetetrazol administration in ovariectomized female rats. Epilepsia. 1986 Mar-Apr;27(2):103–108. doi: 10.1111/j.1528-1157.1986.tb03510.x. [DOI] [PubMed] [Google Scholar]
- Ihara N. A new strain of rat with an inherited cataract. Experientia. 1983 Aug 15;39(8):909–911. doi: 10.1007/BF01990433. [DOI] [PubMed] [Google Scholar]
- Kato S., Ohno K., Ihara N. [Breeding of cataract rat strain (ICRF/Kmu//Yg)]. Jikken Dobutsu. 1990 Apr;39(2):295–298. doi: 10.1538/expanim1978.39.2_295. [DOI] [PubMed] [Google Scholar]
- Lehesjoki A. E., Koskiniemi M., Sistonen P., Miao J., Hästbacka J., Norio R., de la Chapelle A. Localization of a gene for progressive myoclonus epilepsy to chromosome 21q22. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3696–3699. doi: 10.1073/pnas.88.9.3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine D. N., Fisher M. A., Caviness V. S., Jr Porencephaly with microgyria: a pathologic study. Acta Neuropathol. 1974;29(2):99–113. doi: 10.1007/BF00684769. [DOI] [PubMed] [Google Scholar]
- Majewska M. D., Harrison N. L., Schwartz R. D., Barker J. L., Paul S. M. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986 May 23;232(4753):1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- Malafosse A., Leboyer M., Dulac O., Navelet Y., Plouin P., Beck C., Laklou H., Mouchnino G., Grandscene P., Vallee L. Confirmation of linkage of benign familial neonatal convulsions to D20S19 and D20S20. Hum Genet. 1992 Apr;89(1):54–58. doi: 10.1007/BF00207042. [DOI] [PubMed] [Google Scholar]
- Meencke H. J., Janz D. Neuropathological findings in primary generalized epilepsy: a study of eight cases. Epilepsia. 1984 Feb;25(1):8–21. doi: 10.1111/j.1528-1157.1984.tb04149.x. [DOI] [PubMed] [Google Scholar]
- Reigel C. E., Dailey J. W., Jobe P. C. The genetically epilepsy-prone rat: an overview of seizure-prone characteristics and responsiveness to anticonvulsant drugs. Life Sci. 1986 Sep 1;39(9):763–774. doi: 10.1016/0024-3205(86)90454-6. [DOI] [PubMed] [Google Scholar]
- Renier W. O., Renkawek K. Clinical and neuropathologic findings in a case of severe myoclonic epilepsy of infancy. Epilepsia. 1990 May-Jun;31(3):287–291. doi: 10.1111/j.1528-1157.1990.tb05378.x. [DOI] [PubMed] [Google Scholar]
- Sarnat H. B. Disturbances of late neuronal migrations in the perinatal period. Am J Dis Child. 1987 Sep;141(9):969–980. doi: 10.1001/archpedi.1987.04460090046022. [DOI] [PubMed] [Google Scholar]
- Seyfried T. N., Glaser G. H. A review of mouse mutants as genetic models of epilepsy. Epilepsia. 1985 Mar-Apr;26(2):143–150. doi: 10.1111/j.1528-1157.1985.tb05398.x. [DOI] [PubMed] [Google Scholar]
- Sherman G. F., Stone J. S., Rosen G. D., Galaburda A. M. Neocortical VIP neurons are increased in the hemisphere containing focal cerebrocortical microdysgenesis in New Zealand Black mice. Brain Res. 1990 Nov 5;532(1-2):232–236. doi: 10.1016/0006-8993(90)91764-8. [DOI] [PubMed] [Google Scholar]
- Sutula T. P. Experimental models of temporal lobe epilepsy: new insights from the study of kindling and synaptic reorganization. Epilepsia. 1990;31 (Suppl 3):S45–S54. doi: 10.1111/j.1528-1157.1990.tb05859.x. [DOI] [PubMed] [Google Scholar]
- Uga S., Ihara N. Morphological study of a hereditary rat cataract. Exp Eye Res. 1990 Jun;50(6):665–670. doi: 10.1016/0014-4835(90)90111-7. [DOI] [PubMed] [Google Scholar]
- Yamada J., Serikawa T., Ishiko J., Inui T., Takada H., Kawai Y., Okaniwa A. Rats with congenital tremor and curled whiskers and hair. Jikken Dobutsu. 1985 Apr;34(2):183–188. doi: 10.1538/expanim1978.34.2_183. [DOI] [PubMed] [Google Scholar]