Abstract
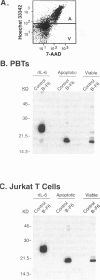
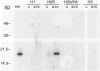
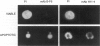
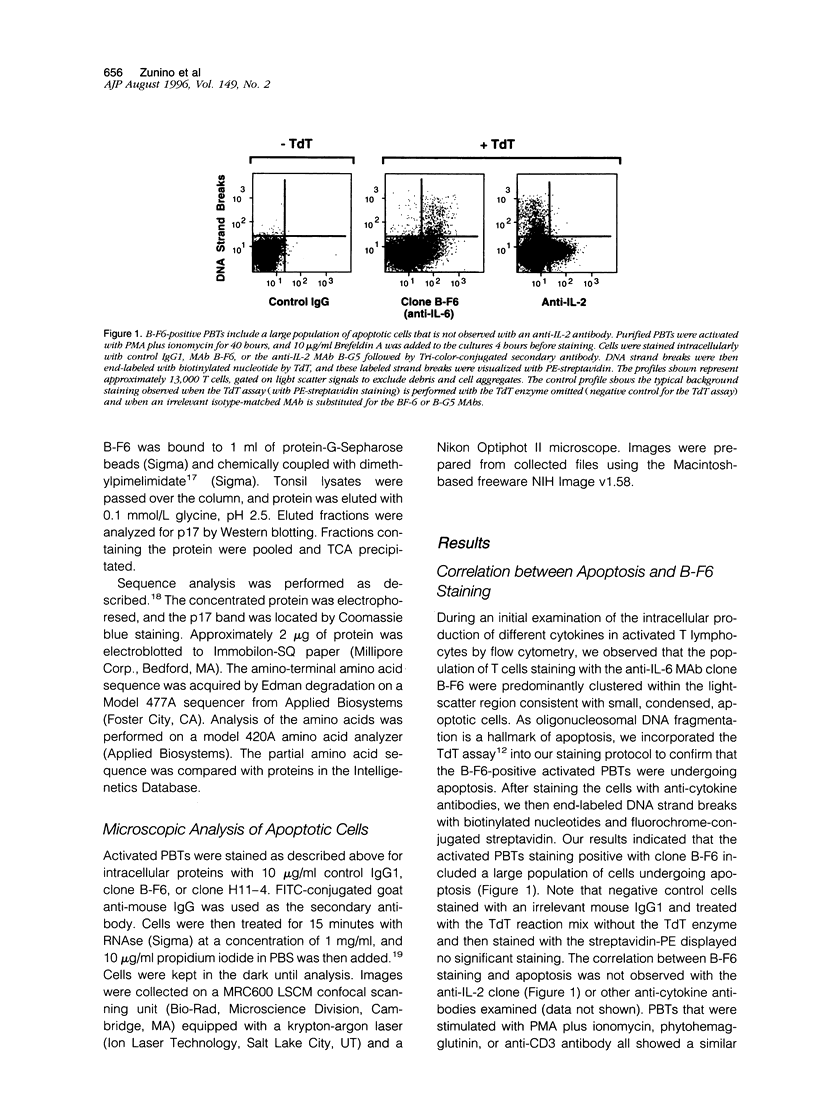
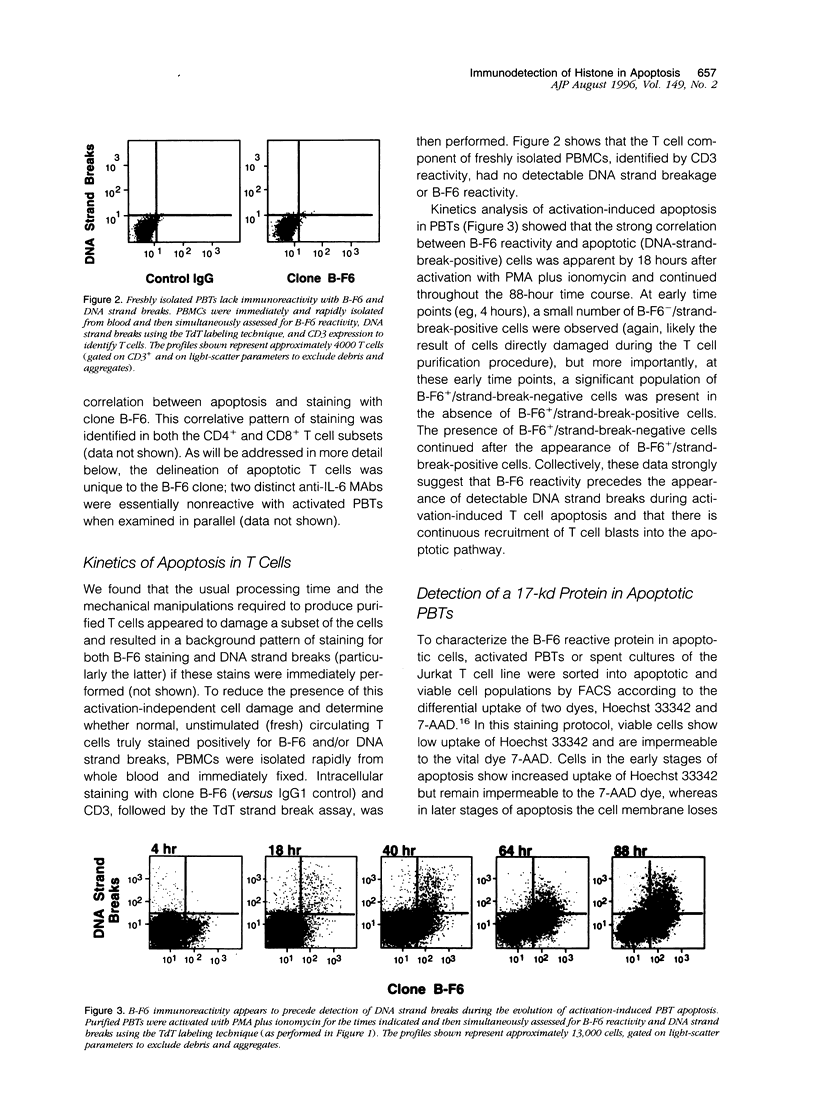
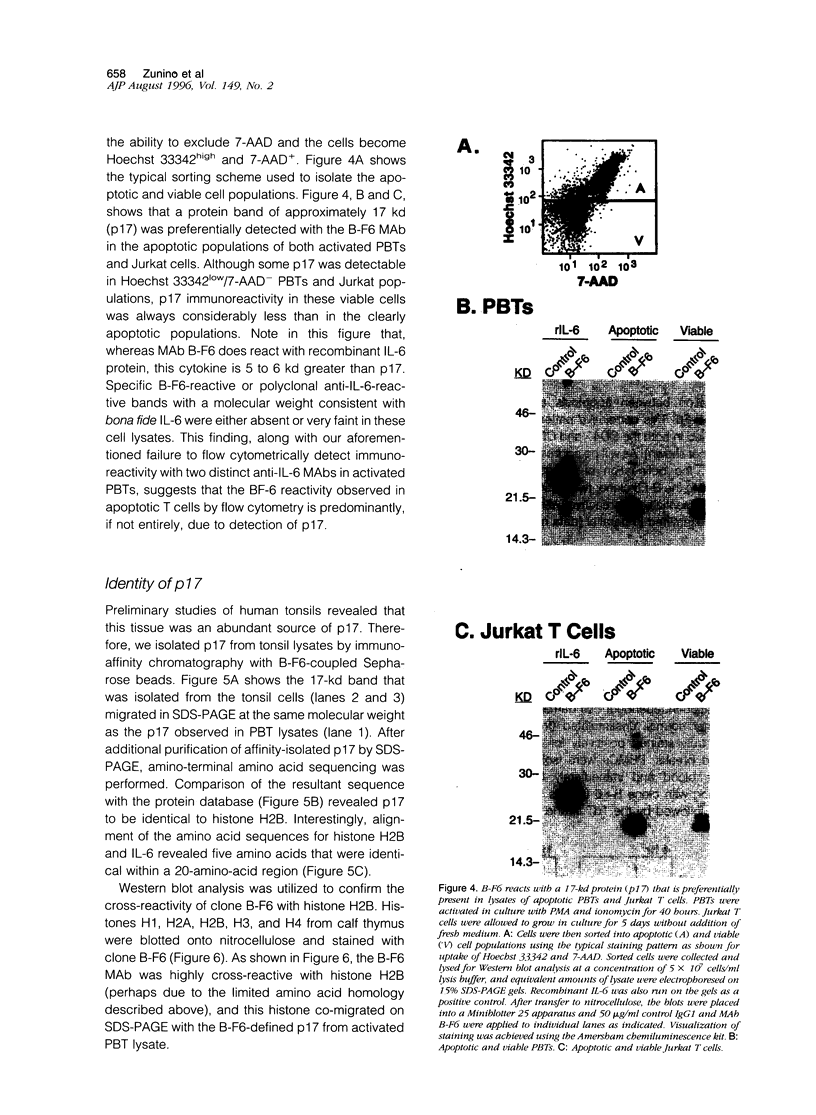
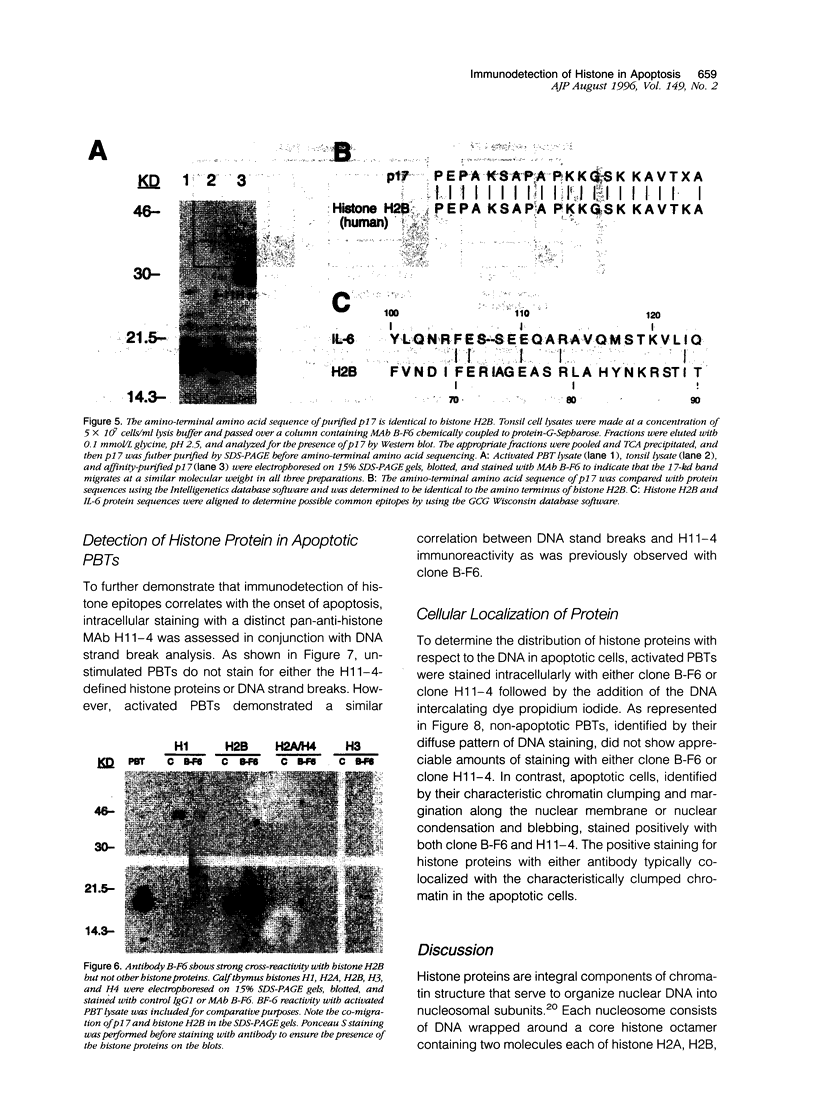
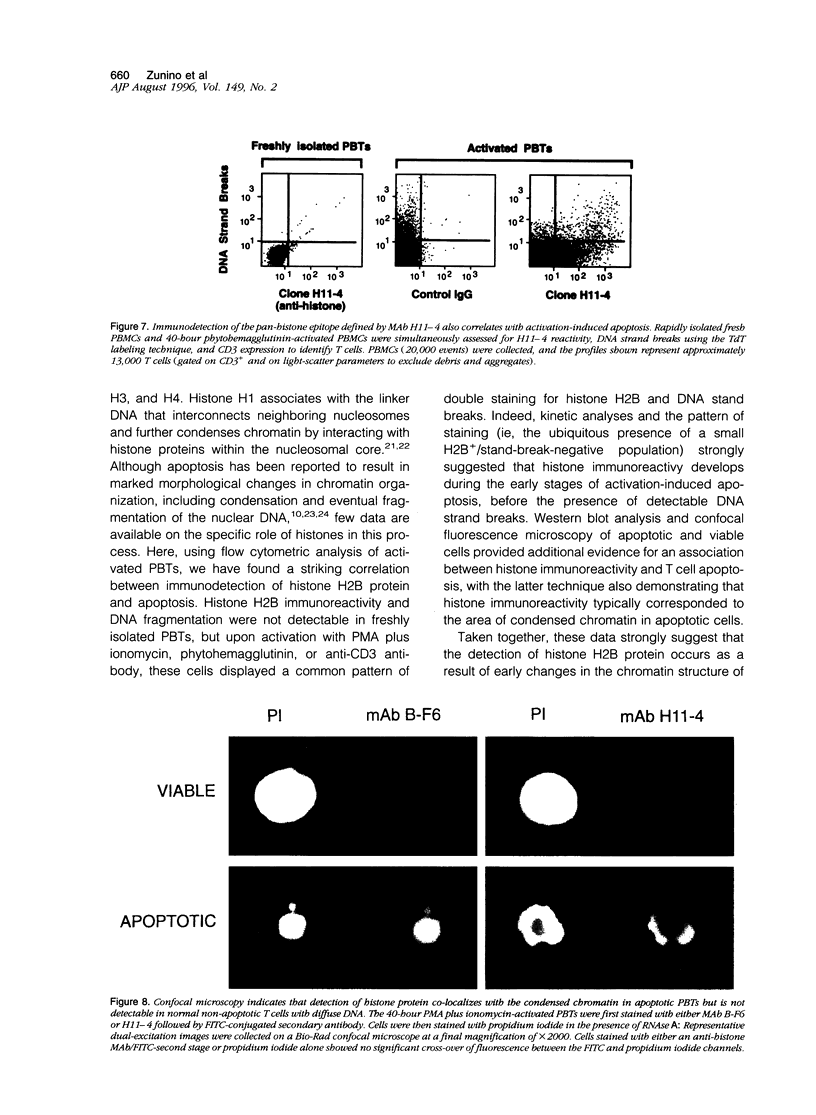
By coupling intracellular staining with terminal deoxynucleotidyl transferase (TdT)-mediated labeling of internucleosomal DNA strand breaks in a flow cytometric assay, we observed a strong correlation between apoptosis-associated DNA strand breaks and immunoreactivity with the monoclonal antibody (MAb) B-F6 in activated human peripheral blood T lymphocytes (PBTs). Although MAb B-F6 has been reported to be specific for the cytokine interleukin-6, Western blot analysis of activated PBT lysates revealed that the predominant protein band detected by this MAb was 17 kd (p17), distinct from the 23-kd core protein and 26- to 30-kd mature glycosylated forms of interleukin-6. Immunoaffinity isolation and amino-terminal amino acid sequence analysis of p17 revealed identity with the histone H2B, a finding confirmed by Western blot analysis of purified histones and by similar staining of activated PBTs with an unrelated anti-histone MAb. Neither histone staining nor DNA strand breakage was observed in freshly isolated PBTs; however, after T cell activation, histone immunoreactivity appeared to precede the appearance of DNA strand breaks, with both increasing to a maximal level by day 3 after activation. Two-parameter confocal immunofluorescence microscopy of histone and DNA staining confirmed a lack of histone immunoreactivity in viable cells and demonstrated co-localization of histone epitopes with abnormally clumped chromatin in apoptotic cells. These data indicate that alteration of histone epitope accessibility is a marker of early apoptosis and suggest that multiparameter flow cytometric analysis of intracellular epitopes may be a powerful tool in the elucidation of intracellular mechanisms of apoptosis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akbar A. N., Salmon M., Savill J., Janossy G. A possible role for bcl-2 in regulating T-cell memory--a 'balancing act' between cell death and survival. Immunol Today. 1993 Nov;14(11):526–532. doi: 10.1016/0167-5699(93)90181-J. [DOI] [PubMed] [Google Scholar]
- Arends M. J., Morris R. G., Wyllie A. H. Apoptosis. The role of the endonuclease. Am J Pathol. 1990 Mar;136(3):593–608. [PMC free article] [PubMed] [Google Scholar]
- Boulikas T. DNA strand breaks alter histone ADP-ribosylation. Proc Natl Acad Sci U S A. 1989 May;86(10):3499–3503. doi: 10.1073/pnas.86.10.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulikas T. Poly(ADP-ribosyl)ation, DNA strand breaks, chromatin and cancer. Toxicol Lett. 1993 Apr;67(1-3):129–150. doi: 10.1016/0378-4274(93)90051-x. [DOI] [PubMed] [Google Scholar]
- Boulikas T., Wiseman J. M., Garrard W. T. Points of contact between histone H1 and the histone octamer. Proc Natl Acad Sci U S A. 1980 Jan;77(1):127–131. doi: 10.1073/pnas.77.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. G., Sun X. M., Cohen G. M. Dexamethasone-induced apoptosis involves cleavage of DNA to large fragments prior to internucleosomal fragmentation. J Biol Chem. 1993 Feb 15;268(5):3037–3039. [PubMed] [Google Scholar]
- Casciola-Rosen L. A., Miller D. K., Anhalt G. J., Rosen A. Specific cleavage of the 70-kDa protein component of the U1 small nuclear ribonucleoprotein is a characteristic biochemical feature of apoptotic cell death. J Biol Chem. 1994 Dec 9;269(49):30757–30760. [PubMed] [Google Scholar]
- Crispe I. N. Fatal interactions: Fas-induced apoptosis of mature T cells. Immunity. 1994 Aug;1(5):347–349. doi: 10.1016/1074-7613(94)90064-7. [DOI] [PubMed] [Google Scholar]
- D'Adamio L., Awad K. M., Reinherz E. L. Thymic and peripheral apoptosis of antigen-specific T cells might cooperate in establishing self tolerance. Eur J Immunol. 1993 Mar;23(3):747–753. doi: 10.1002/eji.1830230327. [DOI] [PubMed] [Google Scholar]
- Gorczyca W., Gong J., Darzynkiewicz Z. Detection of DNA strand breaks in individual apoptotic cells by the in situ terminal deoxynucleotidyl transferase and nick translation assays. Cancer Res. 1993 Apr 15;53(8):1945–1951. [PubMed] [Google Scholar]
- Huletsky A., de Murcia G., Muller S., Hengartner M., Ménard L., Lamarre D., Poirier G. G. The effect of poly(ADP-ribosyl)ation on native and H1-depleted chromatin. A role of poly(ADP-ribosyl)ation on core nucleosome structure. J Biol Chem. 1989 May 25;264(15):8878–8886. [PubMed] [Google Scholar]
- Janssen O., Wesselborg S., Heckl-Ostreicher B., Pechhold K., Bender A., Schondelmaier S., Moldenhauer G., Kabelitz D. T cell receptor/CD3-signaling induces death by apoptosis in human T cell receptor gamma delta + T cells. J Immunol. 1991 Jan 1;146(1):35–39. [PubMed] [Google Scholar]
- Jung T., Schauer U., Heusser C., Neumann C., Rieger C. Detection of intracellular cytokines by flow cytometry. J Immunol Methods. 1993 Feb 26;159(1-2):197–207. doi: 10.1016/0022-1759(93)90158-4. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H., Desnoyers S., Ottaviano Y., Davidson N. E., Poirier G. G. Specific proteolytic cleavage of poly(ADP-ribose) polymerase: an early marker of chemotherapy-induced apoptosis. Cancer Res. 1993 Sep 1;53(17):3976–3985. [PubMed] [Google Scholar]
- Kaufmann S. H. Induction of endonucleolytic DNA cleavage in human acute myelogenous leukemia cells by etoposide, camptothecin, and other cytotoxic anticancer drugs: a cautionary note. Cancer Res. 1989 Nov 1;49(21):5870–5878. [PubMed] [Google Scholar]
- Kawabe Y., Ochi A. Programmed cell death and extrathymic reduction of Vbeta8+ CD4+ T cells in mice tolerant to Staphylococcus aureus enterotoxin B. Nature. 1991 Jan 17;349(6306):245–248. doi: 10.1038/349245a0. [DOI] [PubMed] [Google Scholar]
- Laurent-Crawford A. G., Krust B., Muller S., Rivière Y., Rey-Cuillé M. A., Béchet J. M., Montagnier L., Hovanessian A. G. The cytopathic effect of HIV is associated with apoptosis. Virology. 1991 Dec;185(2):829–839. doi: 10.1016/0042-6822(91)90554-o. [DOI] [PubMed] [Google Scholar]
- Lazebnik Y. A., Kaufmann S. H., Desnoyers S., Poirier G. G., Earnshaw W. C. Cleavage of poly(ADP-ribose) polymerase by a proteinase with properties like ICE. Nature. 1994 Sep 22;371(6495):346–347. doi: 10.1038/371346a0. [DOI] [PubMed] [Google Scholar]
- Lin R., Cook R. G., Allis C. D. Proteolytic removal of core histone amino termini and dephosphorylation of histone H1 correlate with the formation of condensed chromatin and transcriptional silencing during Tetrahymena macronuclear development. Genes Dev. 1991 Sep;5(9):1601–1610. doi: 10.1101/gad.5.9.1601. [DOI] [PubMed] [Google Scholar]
- Mathis G., Althaus F. R. Periodic changes of chromatin organization associated with rearrangement of repair patches accompany DNA excision repair of mammalian cells. J Biol Chem. 1986 May 5;261(13):5758–5765. [PubMed] [Google Scholar]
- Nicoletti I., Migliorati G., Pagliacci M. C., Grignani F., Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991 Jun 3;139(2):271–279. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- Odaka C., Kizaki H., Tadakuma T. T cell receptor-mediated DNA fragmentation and cell death in T cell hybridomas. J Immunol. 1990 Mar 15;144(6):2096–2101. [PubMed] [Google Scholar]
- Picker L. J., Singh M. K., Zdraveski Z., Treer J. R., Waldrop S. L., Bergstresser P. R., Maino V. C. Direct demonstration of cytokine synthesis heterogeneity among human memory/effector T cells by flow cytometry. Blood. 1995 Aug 15;86(4):1408–1419. [PubMed] [Google Scholar]
- Rault-Leonardon M., Atkinson M. A., Slaughter C. A., Moomaw C. R., Srere P. A. Azotobacter vinelandii citrate synthase. Biochemistry. 1995 Jan 10;34(1):257–263. doi: 10.1021/bi00001a031. [DOI] [PubMed] [Google Scholar]
- Realini C. A., Althaus F. R. Histone shuttling by poly(ADP-ribosylation). J Biol Chem. 1992 Sep 15;267(26):18858–18865. [PubMed] [Google Scholar]
- Schmid I., Uittenbogaart C. H., Giorgi J. V. Sensitive method for measuring apoptosis and cell surface phenotype in human thymocytes by flow cytometry. Cytometry. 1994 Jan 1;15(1):12–20. doi: 10.1002/cyto.990150104. [DOI] [PubMed] [Google Scholar]
- Smith C. A., Williams G. T., Kingston R., Jenkinson E. J., Owen J. J. Antibodies to CD3/T-cell receptor complex induce death by apoptosis in immature T cells in thymic cultures. Nature. 1989 Jan 12;337(6203):181–184. doi: 10.1038/337181a0. [DOI] [PubMed] [Google Scholar]
- Sun X. M., Cohen G. M. Mg(2+)-dependent cleavage of DNA into kilobase pair fragments is responsible for the initial degradation of DNA in apoptosis. J Biol Chem. 1994 May 27;269(21):14857–14860. [PubMed] [Google Scholar]
- Tewari M., Quan L. T., O'Rourke K., Desnoyers S., Zeng Z., Beidler D. R., Poirier G. G., Salvesen G. S., Dixit V. M. Yama/CPP32 beta, a mammalian homolog of CED-3, is a CrmA-inhibitable protease that cleaves the death substrate poly(ADP-ribose) polymerase. Cell. 1995 Jun 2;81(5):801–809. doi: 10.1016/0092-8674(95)90541-3. [DOI] [PubMed] [Google Scholar]
- Webb S., Morris C., Sprent J. Extrathymic tolerance of mature T cells: clonal elimination as a consequence of immunity. Cell. 1990 Dec 21;63(6):1249–1256. doi: 10.1016/0092-8674(90)90420-j. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Kerr J. F., Currie A. R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Morris R. G., Smith A. L., Dunlop D. Chromatin cleavage in apoptosis: association with condensed chromatin morphology and dependence on macromolecular synthesis. J Pathol. 1984 Jan;142(1):67–77. doi: 10.1002/path.1711420112. [DOI] [PubMed] [Google Scholar]