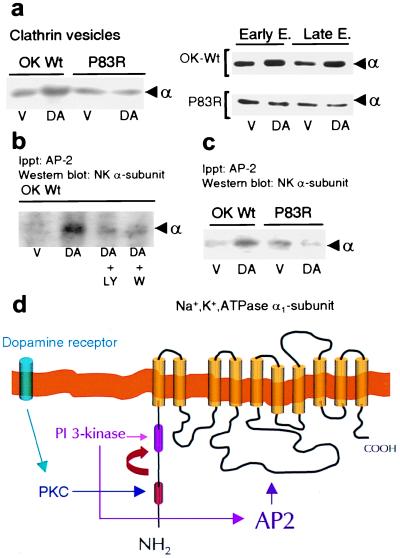
Figure 4.
PI3K activity is required for recruitment and binding of AP-2 to the Na+,K+-ATPase α1 subunit. (a) Na+,K+-ATPase α1 subunit abundance in clathrin vesicles, early endosomes (EE) and late endosomes (LE) from OK cells expressing the wild type (OK-Wt) or P83R mutant. Cells were incubated at 23°C with vehicle (V; Hanks' buffer) or 1 μM DA for either 2.5 min (clathrin vesicles) or 10 min (EE and LE). Experiments were performed at least three times. (b) OK cells incubated with or without 1 μM DA (2.5 min at 23°C) were previously incubated (20 min at 23°C) in the presence or absence of either 100 nM wortmannin or 25 μM LY294002, two inhibitors of PI3K activity. The experiment shown was reproduced with identical results on three separate occasions. In both a and b, the material immunoprecipitated with an AP2αC antibody (Upstate Biotechnology, Lake Placid, NY) was analyzed by Western blotting using an antibody against the Na+,K+-ATPase α1 subunit. (c) OK wild-type (OK Wt) cells and P83R mutants were incubated with or without 1 μM DA (2.5 min at 23°C). Experiments were repeated three times. (d) Organization of the GPCR signals promoting the internalization of Na+,K+-ATPase α1 subunits (see Discussion for details).