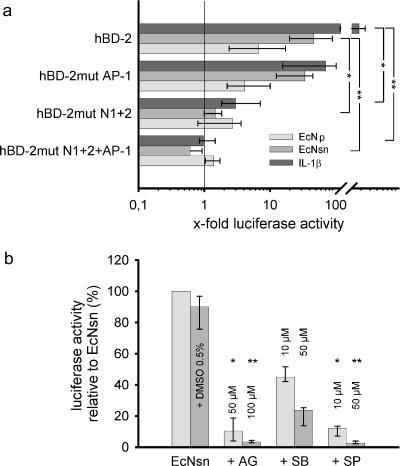
FIG. 2.
Contribution of NF-κB and AP-1 binding sites and MAP kinases for E. coli Nissle 1917 mediated hBD-2 promoter activation. (a) Various mutant promoter constructs were tested for their ability to respond to IL-1β, as well as E. coli Nissle 1917 culture supernatant (EcNsn) or crude bacterial pellet (EcN), in luciferase reporter gene assays as outlined in Fig. 1. NF-κB (positions −205 to −186 and −596 to −572) and AP-1 (positions −127 to −121) were mutated separately (hBD-2mut N1+2 or hBD-2mut AP-1) or in combination (hBD-2mut N1+2+AP-1). Maximal activity was observed with the complete promoter encompassing all known NF-κB and AP-1 sites. Sequential loss of transcription factor binding sites within the hBD-2 promoter resulted in a stepwise decrease in activation upon IL-1β, as well as pellet (EcN) and supernatant (EcNsn) treatment. The data represent the median with minimum and maximum values of duplicate samples of three to four experiments. Statistical evaluation was performed by using Kruskal-Wallis analysis with post hoc Dunn's test. *, P < 0.05; **, P < 0.01. (b) Specific MAP kinase inhibitors (diluted in DMSO) at the indicated concentrations were added 1 h prior to 4.5 h stimulation with bacterial supernatant or the corresponding supernatant, including 0.5% DMSO. Blocking of ERK1/2 by AG126 (AG) and JNK by SP600125 (SP) resulted in almost complete abrogation of E. coli Nissle 1917 supernatant-mediated hBD-2 promoter activation. Inhibition of p38 MAP kinase by SB203580 (SB) had the slightest effect. The data represent the median with minimum and maximum values of duplicate determinations of three experiments. Each inhibitor was compared to the DMSO control by Kruskal-Wallis analysis and post hoc testing. *, P < 0.05; **, P < 0.01.