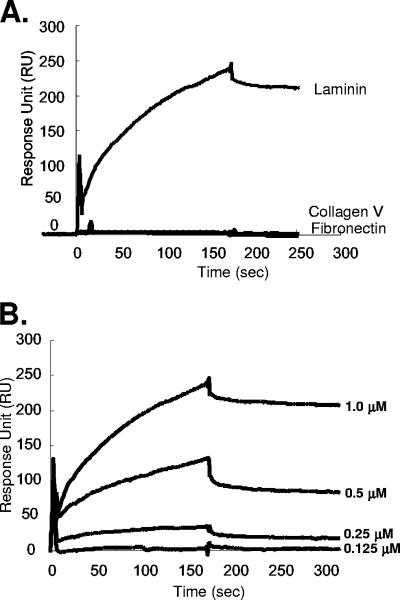
FIG. 7.
(A) SPR analysis of the binding of A26L(127-500) to laminin, collagen V, or fibronectin. Biotinylated GST-A26L(127-500) was immobilized on an SA sensor flow chip and tested for the binding of 1 μM laminin, collagen V, or fibronectin as described in Materials and Methods; the first 180 s represent association, and the next 300 s represent dissociation. (B) SPR sensorgrams of laminin binding after removal of the background binding of laminin to biotinylated GST using four different concentrations of laminin (0.125, 0.25, 0.5, and 1.0 μM) with a flow rate of 10 μl/minute to extract the association/dissociation kinetics parameters. As a result, a kon of 1.5 × 104 M−1 s−1, a koff of 2.0 × 10−4 s−1, and a KA (=kon/koff) of 7.5 × 107 M−1 were determined for the A26L and laminin interaction (χ2, 9.6). The binding rate constants were derived from a 1:1 Langmuir binding model (1). The association/dissociation kinetics were measured and recorded by SPR as described in Materials and Methods.