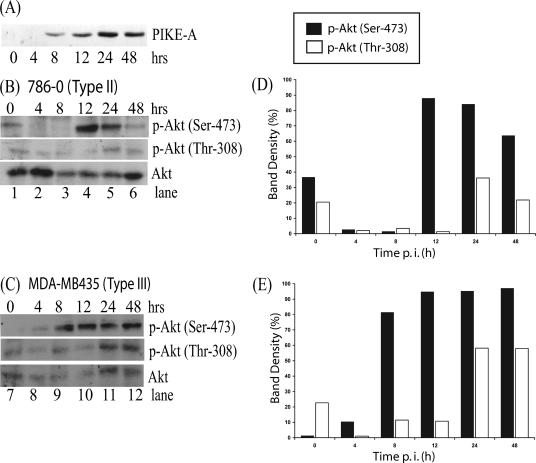
FIG. 3.
Induction of endogenous Akt phosphorylation following transfection of PIKE-A in human cancer cells. (A) HEK 293 cells were transfected with the PIKE-A plasmid, and expression was detected at various time points by Western blotting with an anti-Myc antibody. PIKE-A plasmid was transfected into (B) 786-0 and (C) MDA-MB435 cells, and Akt phosphorylation at both p-Akt Ser-473 and p-Akt Thr-308 sites was detected in cell lysates (50 μg per lane) by Western blotting at various times following transfection. The levels of Akt phosphorylation at Ser-473 and Thr-308 were determined with Molecular Imaging software (Kodak) and compared to the protein level of Akt to quantify the stimulation induced by overexpression of PIKE-A in (D) 786-0 and (E) MDA-MB435 cells. Immunoblot signal variability between films was normalized.