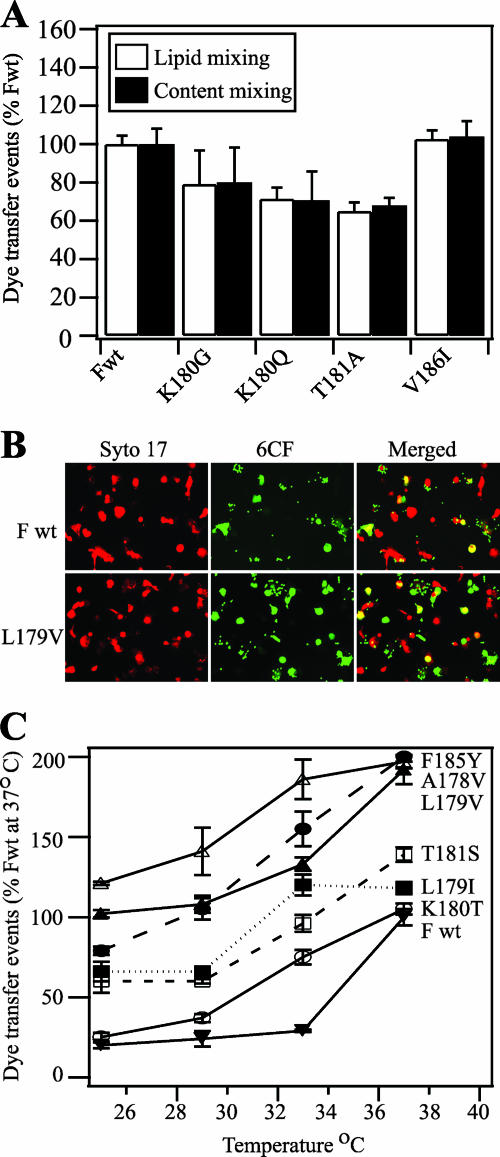
FIG. 6.
Dye transfer assays of SeV F protein-mediated membrane fusion. (A) Lipid mixing (white bars) and content mixing (black bars) by SeV F proteins. Effector CV-1 cells were infected with vaccinia virus vTF7.3 and transfected with 1 μg each of SeV F DNA (wild-type [Fwt] or mutant) and SeV HN DNA. Target RBCs were colabeled with the lipidic dye R18 and the aqueous dye 6CF. At 16 h posttransfection, CV-1 cells were incubated with TPCK-treated trypsin (5 μg/ml) for 1 h at 37°C. Effector CV-1 cells were coincubated with target RBCs for 1 h at 4°C and incubated for 15 min at 37°C to promote dye transfer. The means and standard errors from three microscopic fields are reported. (B) Representative, cropped one-quarter-field images of dye transfer mediated by SeV Fwt or SeV F L179V. Effector CV-1 cells were infected and transfected as described above. At 16 h posttransfection, CV-1 cells were incubated with TPCK-treated trypsin (5 μg/ml) for 1 h at 37°C. Effector CV-1 cells were labeled with the nucleic acid dye SYTO 17 (red). RBC target cells were labeled with 6CF (green) only. Cell-cell fusion was monitored by the transfer of 6CF dye from the RBCs to the CV-1 effector cells (yellow). (C) Temperature dependence of SeV F-mediated dye transfer coexpressed with the SeV HN protein. CV-1 effector cells and RBC target cells were labeled and coincubated as described for panel B. At 16 h posttransfection, CV-1 cells were incubated with TPCK-treated trypsin (5 μg/ml) for 1 h at 37°C. After coincubation of effector and target cells at 4°C for 1 h, the cell complexes were incubated for 15 min at 25, 29, 33, or 37°C. Cell-cell fusion was monitored as described for panel B. Dye transfer events are normalized to SeV Fwt at 37°C. The means and standard errors from four microscopic fields are reported.