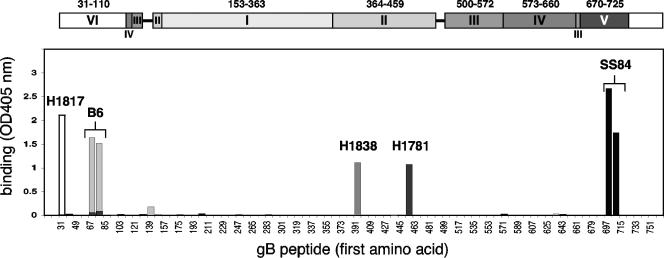
FIG. 3.
Peptide mapping of MAb to gB by ELISA. Biotin-coupled overlapping peptides mimicking the gB sequence were immobilized on a 96-well plate coated with streptavidin and incubated with 20 μg of purified IgG/ml. Bound IgG was revealed with anti-mouse peroxidase and substrate (ABTS). The absorbance was read at 405 nm. For simplicity, the graph is a composite result of separate experiments with the four MAbs indicated. On the x axis, the numbers refer to the first amino acid of the peptide sequence, and every other peptide is indicated. At the top, the domain architecture of gB is shown in the scale of the x axis. Bar graph binding profiles for MAb H1817 (white), B6 (light gray), H1838 (gray), H1781 (dark gray), and SS84 (black) are presented.