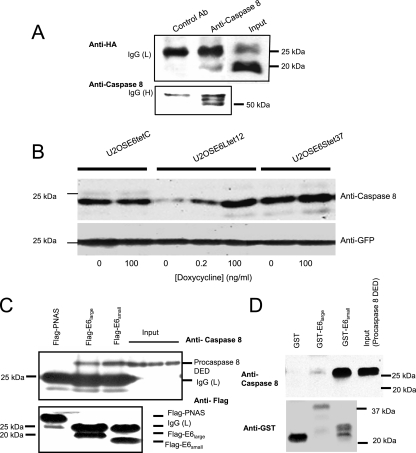
FIG. 6.
E6 binds to procaspase 8. (A) E6 coimmunoprecipitates with endogenous procaspase 8. U2OSE6tet24 cells (2 × 107) grown in the absence of doxycycline (and hence expressing E6) were lysed with the immunoprecipitation buffer. Five micrograms of irrelevant, isotope-matched control antibodies (left lane) or anti-caspase 8 monoclonal antibodies (middle lane) was used to precipitate a complex which was then bound to Plus A/G agarose. The bound proteins were eluted and subjected to immunoblot analysis. The detection of HA-E6 in the coimmunoprecipitated samples and in the input sample (right lane) was performed using anti-HA rat polyclonal antibodies (top). The same membrane was stripped and reblotted with anti-caspase 8 polyclonal antibodies as a control for precipitation (bottom). IgG (H) and (L), immunoglobulin G heavy and light chains. (B) E6large causes a decrease in the level of exogenously expressed procaspase 8 DED. The indicated cell lines were grown in the indicated concentrations of doxycycline and transiently transfected with pcDNA3-caspase 8 DED and pcDNA3-GFP 48 h prior to lysis. The transfection efficiency was estimated and normalized using anti-GFP antibodies (bottom). (C) Procaspase 8 binds to both E6large and E6small/E6* as analyzed by coimmunoprecipitation. U2OS cells were cotransfected with pFlag-LOC5129 (negative control), pFlag-E6large, or pFlag-E6small/E6* and pTriEX4-procaspase 8 DED. Forty-eight hours posttransfection, the expressed LOC5129-tagged proteins were precipitated using Flag-agarose. The bound proteins were then subjected to separation by SDS-PAGE and immunoblotting with antibodies directed against either caspase 8 (top) or Flag (bottom). The three right lanes show the input levels of procaspase 8 for the incubations shown in the three left lanes. (D) Both HPV-16 E6large and HPV-16 E6small/E6* bind to procaspase 8 DED produced by in vitro transcription/translation, as analyzed by in vitro pull-down assays. Procaspase 8 DED, expressed using the T7 TNT Quick Coupled Transcription/Translation System, was incubated with beads bound to either GST, GST-E6large, or GST-E6small/E6*. The beads were washed, and the bound proteins were eluted with SDS prior to separation by SDS-PAGE and immunoblotting with antibodies directed against procaspase 8 (top) or GST (bottom). An amount of lysate corresponding to the input was also loaded as a control (far right lane).