Figure 1.
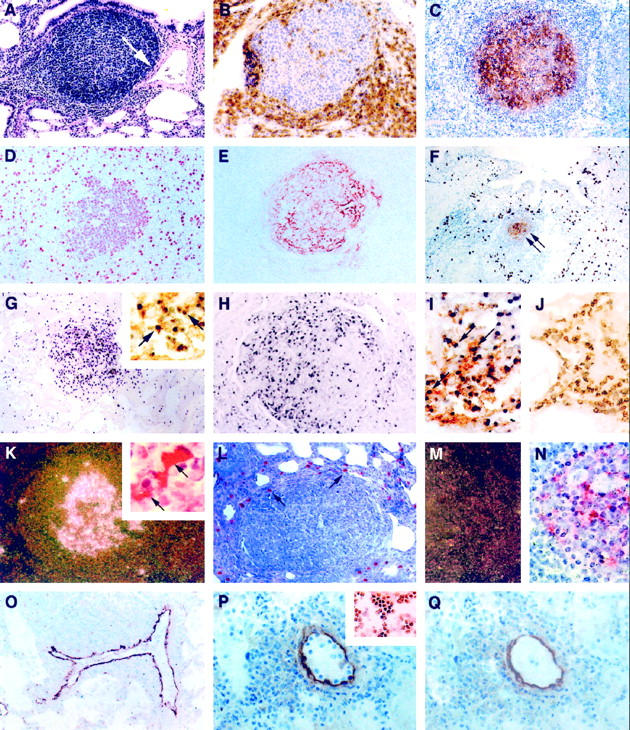
Infiltrative and proliferative lesions associated with pediatric lymphocytic interstitial pneumonia. Immunoperoxidase (IP) procedures used 3,3′-diaminobenzidine as the chromogen, and in situ hybridization (ISH) procedures used anti-digoxigenin-alkaline phosphatase (αDIG-AP) or 125I-labeled CTP as an indicator. A: Widespread thickening of the pulmonary interstitium with mononuclear leukocytes and a single peribronchiole and perivascular aggregate of lymphocytes. Note that the expansion of lymphocytes is associated with constriction of a medium-sized artery (arrow) H&E, magnification, ×100. B: Perifollicular aggregation of CD8+ T lymphocytes. IP, CD8 (clone C8/144B, DAKO); magnification, ×250. C: Lymphoid aggregate showing a predominance of immature B cells within the germinal center. IP, CD19 (clone 4G7/2E, Novocastra, Newcastle, UK); magnification, ×100. D to F: Most germinal center lymphoblasts were mitotically active (D; IP, PCNA (clone PC10, DAKO); magnification, ×250) and surrounded well developed networks of FDC (E and F, arrows; IP, CD21 (clone 1F8, DAKO); magnification, ×100). F: Alveolar and interstitial macrophages were found in high numbers on the periphery of lymphoid follicles. IP, CD68 (KP1, DAKO); magnification, ×100. G: A high percentage of cells (>20%) within the follicular germinal center of lymphoid aggregates contained high copy numbers of EBV EBER-1 RNA. ISH αDIG-AP for EBER-1; magnification, ×100. Most of these EBV RNA-positive cells were of B cell lineage (G, inset; combined ISH αDIG-AP for EBER-1 and IP for CD20; magnification, ×400), and many expressed EBV latent membrane proteins (H, IP for EBV LMP (clone CS1–4, DAKO); magnification, ×200). I: EBV also co-localized to follicular dendritic cells (arrows; combined ISH αDIG-AP for EBV RNA and IP for CD21 FDC; magnification, ×400), but not to interstitial T lymphocytes J; combined ISH αDIG-AP for EBV RNA and IP for CD3; magnification, ×400). K: HIV mRNA was observed within follicular germinal centers (125I ISH for HIV-1 mRNA, dark-field; magnification, ×40) in association with FDC (combined 125I ISH for HIV-1 mRNA and IP for CD21 FDC; inset, ×1000); however, cells that expressed viral antigens were found mostly on the periphery of lymphoid aggregates in areas normally occupied by macrophages (L, arrows; IP for HIV gag p24 (clone Kal-1, DAKO; magnification, ×200). M and N: Serial tissue sections hybridized with noncomplementary HIV RNA probes transcribed in sense-strand orientation did not form hybrids (M, 125I ISH for HIV-1 RNA, dark-field; magnification, ×40; N, 125I ISH for HIV-1 RNA; magnification, ×500). O: VCAM-1 expression was heightened in vessels with perivascular inflammatory foci. IP, CD106 (clone 51–10C9, PharMingen); magnification, ×100. P: Tissue adhesion assay demonstrating that CD8+ T cell clones that co-express the VLA-4 ligand (inset, IP for VLA-4α4/β1; magnification, ×200) selectively adhere to venular endothelium in the lung from a child with severe LIP (IP for VCAM-1; magnification, ×400). Q: Pretreatment of sequential tissue sections (shown in P) with blocking antibodies to VCAM-1 and incubation of CD8+ T cell clones with blocking antibodies to VLA-4α4/β1 inhibited >80% of this adhesion. After the adhesion assay, the tissues were stained with antibody to VCAM-1 to demonstrate presence of VCAM-1 expression. IP for VCAM-1; magnification, ×400.
