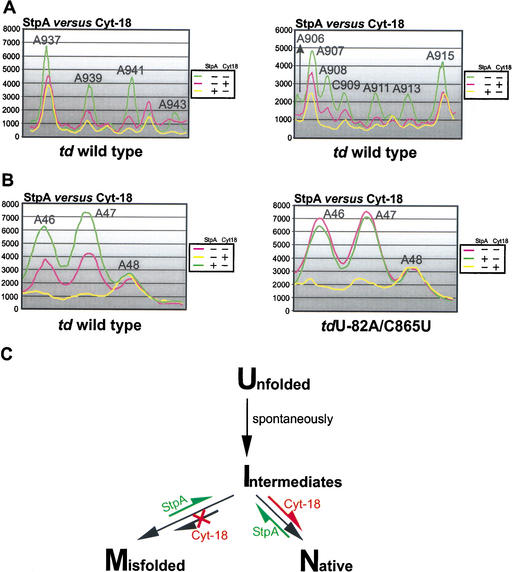
Figure 6.
Opposite effects of StpA and Cyt-18. (A) The opposite effects of these proteins on the accessibility of the residues in J8/7 (left panel) and of bases, which are close to or part of the loop E motif in the P7.1-P7.2 extension (right panel), to DMS are summarized. (B) Comparison of the accessibility to DMS of the A46–A48 in the presence and absence of either StpA and Cyt-18 in the context of the wild-type td intron (left panel) and the mutant tdU-82A/C865U (right panel). (C) A model for the mechanisms of action of the RNA chaperone StpA and the group I intron-specific splicing factor Cyt-18. The equilibrium shifts within the folding pathway induced by either StpA or Cyt-18 are marked in green or red. U, unfolded molecules, having formed the secondary but not the tertiary structure; M, misfolded molecules, secondary and tertiary structure of the intron are formed but alternative mispaired conformations at the 2D or 3D level occur within the pre-mRNA; I, intermediate molecules, secondary and part of the 3D structure are formed, but these molecules still lack the formation of long-range tertiary interactions; and N, molecules with a native 3D fold having a stable, productive structure leading to splicing competent, functional RNA.