Abstract
Pulmonary tumorlets are minute neuroendocrine cell proliferations believed to be precursor lesions to pulmonary carcinoids. Little is known of their molecular pathogenesis because of their small size. Using tissue microdissection, we evaluated 11q13 region allelic imbalance in the pathogenesis of pulmonary tumorlet/carcinoid lesions. The int-2 gene was selected because of its chromosomal location at 11q13 in close proximity to MEN1, a tumor suppressor gene frequently mutated in familial forms of neuroendocrine cancer. Three cohorts of patients were studied: subjects with typical carcinoid tumors and coexisting tumorlets (n = 5), typical carcinoids without tumorlets (n = 6), and tumorlets alone without carcinoid lesions (n = 5). A total of 11 carcinoids and 11 tumorlets were microdissected from 4-μm-thick histological sections. Genotyping was designed to detect allelic imbalance of the int-2 gene and involved DNA sequencing of two closely spaced deoxynucleotide polymorphisms. Subjects shown to be informative were evaluated for allelic imbalance in tumorlet/carcinoid tissue. Eight of 11 (73%) carcinoids manifested allelic, in contrast to only one of 11 (9%) of tumorlets. Int-2 allelic imbalance was significantly associated with carcinoid tumor formation (P < 0.01). In patients having both carcinoid tumors and tumorlets, the latter showed allelic balance and were thus discordant in genotype with coexisting carcinoid excluding pathogenesis of tumorlets from intramucosal spread from carcinoid tumors. Int-2 allelic imbalance was shown to be an early event in carcinoid tumor formation by virtue of the absence of allelic imbalance for other common cancer-related gene disturbances involving 11p13 (Wilms’ tumor), 3p25 (von-Hippel-Lindau), and 17p13 (p53). Demonstration of 11q13 allelic imbalance by microdissection/genotyping may be a useful discriminatory marker for pulmonary neuroendocrine neoplasia.
Neoplastic entities sharing similar histogenesis can be difficult to distinguish on morphological grounds, especially when specimen size is small or otherwise limited. Differential diagnosis based on objective genetic criteria would be a valuable addition in this context, serving as an independent parameter for tissue diagnosis. An example in this regard is the distinction between pulmonary tumorlet and typical carcinoid tumor. They share virtually identical histopathological features, differing primarily in size. From a biological perspective, however, the tumorlet is usually benign and silent, whereas the carcinoid tumor has the potential to grow and metastasize and can be associated with paraneoplastic sequelae.
The relationship between carcinoid tumor and tumorlet has not been definitively established, although it is held that the former evolves from the latter as part of a process of neoplastic progression. 1 This evolution is likely based on the acquisition of critical cancer-related genetic alterations which, if understood, would serve as the basis for distinguishing the two entities. Tumorlets are significantly more frequent than carcinoid tumors and often are multifocal and bilateral, 2 suggesting the presence of a “field effect” for their formation. The vast majority remain as incidental lesions and do not progress beyond the tumorlet lesion. 1,2 Alternatively, the finding of one or more pulmonary tumorlets in a case of carcinoid tumor raises the possibility that the former may in fact represent intramucosal metastatic dissemination from the latter. In this situation, the tumorlet deposit would be expected to possess the genotypic alterations already present in the carcinoid tumor. The pathogenetic relationship between tumorlet and carcinoid may be critically evaluated by an approach involving comparative genetic analysis of carefully selected tissue representative of each entity in an individual case.
Molecular markers used in the context of differential diagnosis in surgical pathology must first take into account the need for accurate sample selection as a basis for correlative genetic analysis. This requires histologically guided sample selection performed after thorough microscopic evaluation. In addition, the genotyping methodology must be effective on relatively small sample sizes, independent of fixation character and age of archival storage. It must also be reliable and simple in technical performance to be of general application. Given the close relationship between tumor suppressor gene loss and oncogene amplification with neoplastic development and progression, 3 characterization of representative microdissected tumor samples for cancer-related gene loss or gain may constitute a useful differential genotyping approach. This concept formed the basis for the experimental design in this study evaluating two closely related forms of pulmonary neuroendocrine neoplasia.
Several recently characterized cancer gene alterations have been implicated in the development and progression of neuroendocrine cell neoplasia. 4-15 In many cases candidate genes have been discovered through the study of inherited forms of endocrine cancer. 4-7,16-18 Most notably, the MEN1 gene, situated at 11q13, has been closely linked to familial forms of multiple endocrine neoplasia. 4,16,17 MEN1 is believed to have tumor suppressor gene properties and has been shown to undergo deactivating point mutations in both familial and sporadic endocrine tumors. 17,19 Further research on the role of this gene in endocrine neoplasia is likely to yield important insights into the mechanism of tumor development with the potential for clinical translation into diagnostic testing. The evidence to date provides strong support for this gene in the evaluation of neuroendocrine neoplasia.
Tumor suppressor genes such as MEN1 are believed to operate through a two-stage process of gene damage as described by Knudsen. 20 Inactivating point mutations of one allele may be followed in time by deletion of the remaining normal gene copy to create a significant loss of tumor suppressor gene activity within the affected cell. Whereas the former is difficult to assess because of the need for extensive analysis of gene sequences, the latter can be more readily accomplished by searching for loss of heterozygosity, using one or more intragenic polymorphic DNA sequences and/or microsatellite markers for a particular gene of interest. This approach, searching for allelic imbalance (loss of heterozygosity) with respect to a specific tumor suppressor gene, has been successfully applied to a wide range of growth regulatory genes and tumor types. 21 Allelic imbalance analysis requires, however, the knowledge of an informative polymorphic marker for a particular gene of interest. Given the availability of such an informative DNA polymorphism, the assessment can then be easily extended to microdissected tissue samples, based on the use of polymerase chain reaction (PCR) to generate adequate amounts of DNA for allele content determination. 22
Unfortunately, detailed knowledge of polymorphic sites within the MEN1 gene is not readily available at this time, precluding direct determination of gene loss. A reasonable alternative is to evaluate a gene situated close to the MEN1 gene at chromosome 11q13. The int-2 gene would appear to be an appropriate candidate in this regard. 24,25 Several DNA sequence polymorphisms have been described in the literature. 25,26 In this study, therefore, we undertook allelic imbalance analysis of int-2 as a measure of 11q13 gene loss in evaluating the pathogenesis and potential clinical utility for discriminating between pulmonary tumorlets and typical carcinoid tumors.
Materials and Methods
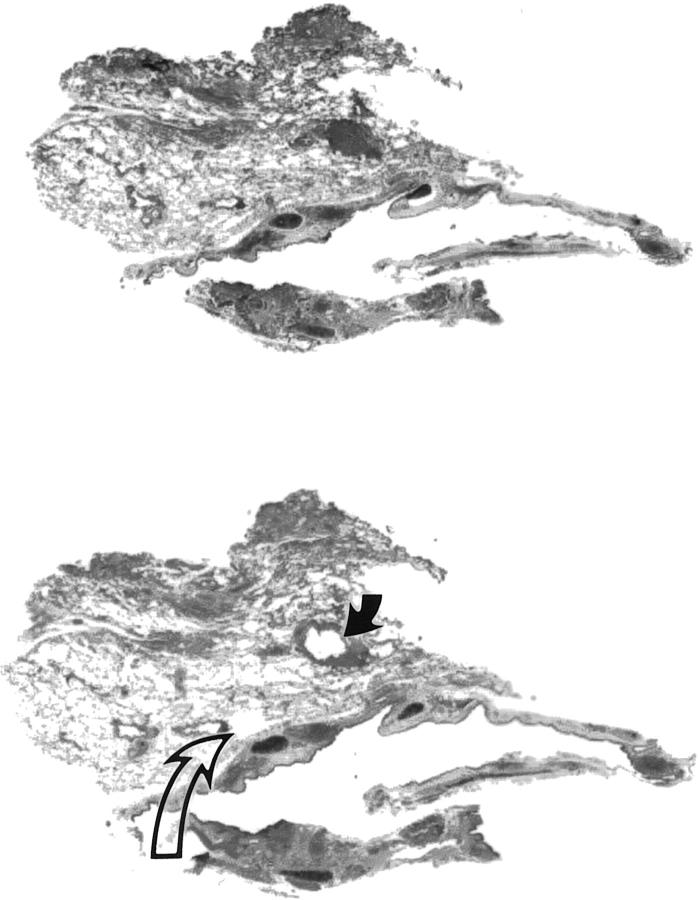
Sporadic typical pulmonary carcinoid and/or tumorlet specimens were obtained from formalin-fixed, paraffin-embedded tissue archives of 26 subjects from the University of Pittsburgh Medical Center and the Mayo Clinic (Scottsdale, AZ). Subjects were assigned to one of three groups: sporadic typical carcinoid tumor only (n = 10), sporadic typical carcinoid tumor with one or more associated tumorlets (n = 8), and tumorlet only (n = 8). Atypical carcinoid tumors as well as high-grade small or large cell neuroendocrine cancers were excluded from the study. Criteria for atypical carcinoid tumor were mitotic rate over two per 10 high-power fields and presence of necrosis. Tissue targets for genotyping consisted of normal lung, carcinoid tumor, and tumorlet, selected on the basis of histopathological review of hematoxylin-eosin-stained slides. Tissue microdissection was performed as previously described, 27,28 using the aggregate material from one to three 4-μm-thick unstained histological sections (Figure 1) ▶ . No familial cases were included in this study.
Figure 1.
Microdissection of pulmonary tumorlet. Top: Premicrodissection. Bottom: Postmicrodissection. Microdissected areas range in size from 1 to 3 mm in greatest dimension. Normal pulmonary parenchyma was removed to serve as a internal negative control (open arrow). Note that the normal tissue sample for genotyping (open arrow) is similar in size to that of the tumorlet (solid arrow). Aggregate tissue was collected from up to four blank histological sections. Hematoxylin-eosin, magnification ×10.
Pulmonary tumorlets are microscopic lesions, usually manifesting as a spindle cell proliferation arising in a bronchiole in association with the bronchovascular bundle. They measured no more than 3 mm along their greatest dimension. Typical carcinoid tumors were all greater than 10 mm in diameter and displayed a spectrum of epithelial and spindle cell growth patterns typical of this type of neoplasm. With representative tissue blocks selected for genotyping, one to three 4-μm-thick histological sections were prepared for microdissection. In seven instances, once in a patient with coexisting carcinoid tumor and tumorlet and the remainder in patients with tumorlets only, recut microscopic section no longer contained tumorlet tissue. This was attributed to the small size of tumorlets and tissue loss related to preparation of recut histological sections.
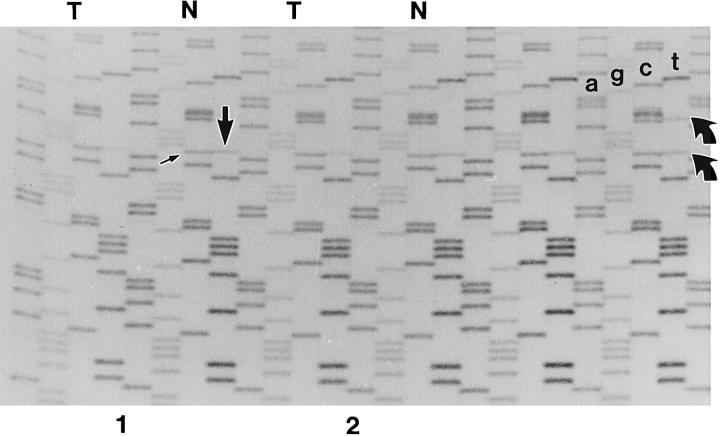
Allelic imbalance in the region of 11q13 was based on using a pair of closely spaced DNA sequence polymorphisms in the second intron of the int-2 gene. 26 For each subject, the normal lung tissue sample was amplified across the polymorphic region and sequenced in the manner previously described. 29,30 Oligonucleotide primers for amplification and sequencing of intron 2 of the int-2 gene were 5′-gtctggcctgcacatcaag-3′ (sense) and 5′-cagcctctaacagacacag′3′ (antisense). Subjects found to be noninformative for sequence polymorphisms could not be evaluated for int-2 allelic imbalance. In informative subjects, the intensity ratio of the polymorphic DNA sequencing bands, as assessed by visual observation, was required to be half intensity and equal to qualifying the case for tumorlet/carcinoid allelic imbalance determination (Figure 2) ▶ . Allelic imbalance was determined to be not present when neoplastic samples demonstrated isointense polymorphic DNA sequencing bands, as in the case of normal lung tissue. Allelic imbalance was determined to be present when polymorphic bands of neoplastic samples where not isointense (Figure 2) ▶ . In a similar fashion, allelic imbalance was assessed at 3p25, 11p13, and 17p13.1 in selected cases by evaluating DNA sequence or microsatellite polymorphisms within the von Hippel–Lindau, 31 Wilms’ tumor, 32 and p53 33 tumor suppressor genes.
Figure 2.
int-2 allelic imbalance genotyping. Autoradiograms of DNA sequencing gels are analyzed at point of DNA sequence polymorphisms in intron 2 of the int-2 gene. The photograph contains the sequencing results from six samples. In the sample to the far right representing normal pulmonary parenchyma, the subject demonstrates both DNA sequence polymorphisms (curved arrows) (see text and Ref. 26 for details). The four DNA sequencing ladders to the left represent carcinoid tumor (T) and normal pulmonary parenchyma (N) from two different subjects. Allelic imbalance is demonstrated by uneven intensity of polymorphic bands (straight arrows) in comparison to that evident in respective normal parenchyma (see Materials and Methods for details).
Results
Pulmonary tumorlets were small lesions (usually less than 0.3 cm in diameter) that arose in or adjacent to bronchovascular bundles. The proliferating cells tended to have a spindle shape and formed intraepithelial aggregates beneath an intact ciliated bronchiolar mucosa. Extension into the underlying submucosa was characteristic, with the spindle cells forming nests and short fascicles within an expanded submucosa. Cytologically, the cells had elongated nuclei with “salt and pepper” chromatin and bipolar, lightly eosinophilic cytoplasm. Mitoses were absent.
Typical carcinoids ranged in size from 1.5 to 8.0 cm (mean 2.2 cm). Sixteen tumors comprised polygonal cells with round to oval nuclei, “salt and pepper” chromatin, and abundant granular eosinophilic cytoplasm. Most showed a mixture of Zelballen, trabecular, or insular growth, although a spindle growth pattern was seen in three cases. Two tumors had predominantly spindle growth corresponding to so-called peripheral carcinoid tumors. In all cases a delicate branching network of congested and compressed capillaries separated the epithelial nests. Mitotic rates were less than one mitosis per 20 high-power fields.
Because of the small size of tumorlets, in was not infrequent (n = 7) to fail to identify them in recut histological sections for microdissection-based genotyping (Table 1) ▶ . Despite the small size of tumorlets, no difficulties were encountered in accurately sampling such lesions by hand under stereomicroscopic observation (Figure 1) ▶ . It should be noted, however, that absolute exclusion of normal cells could not be achieved by means of manual microdissection, as inevitably small numbers of mesenchymal support cells were gathered together with the neoplastic tissue. Nevertheless, the enrichment with target cells was such as to render the contamination a minor component (Figure 1) ▶ .
Table 1.
Microdissection-Based Genotyping for Allelic Imbalance: Pulmonary Tumorlet-Carcinoid Series
| Patient | Tumor type | Int-2 11q13.1 | p53 17p13.1 | VHL 3p25 | WT-1 11p13 |
|---|---|---|---|---|---|
| Carcinoid with tumorlets (3 patients noninformative for int-2) | |||||
| 1 | Carcinoid | + | − | − | − |
| Tumorlet | − | − | − | − | |
| 2 | Carcinoid | + | − | NI | NI |
| Tumorlet | − | − | NI | NI | |
| 3 | Carcinoid | + | − | NI | |
| Tumorlet | − | − | NI | ||
| 4 | Carcinoid | + | NI | − | |
| Tumorlet | − | NI | − | ||
| 5 | Carcinoid | − | |||
| (Tumorlet)* | |||||
| Carcinoid only (4 patients noninformative for int-2) | |||||
| 1 | Carcinoid | + | − | NI | − |
| 2 | Carcinoid | + | − | NI | − |
| 3 | Carcinoid | + | − | − | NI |
| 4 | Carcinoid | + | NI | ||
| 5 | Carcinoid | − | NI | ||
| 6 | Carcinoid | − | |||
| Tumorlet only (3 patients noninformative for int-2) | |||||
| 1 | Tumorlet | − | − | NI | − |
| 2 | Tumorlet | − | NI | NI | |
| 3 | Tumorlet | + | − | NI | |
| 4 | Tumorlet | − | − | − | NI |
| Tumorlet | − | − | − | NI | |
| 5 | Tumorlet | − | NI | − | |
| Tumorlet | − | NI | − |
*Seven tumorlets (one from the carcinoid and tumorlet group, six from the tumorlet-only group) identified on hematoxylin-eosin staining could not be found on recut unstained histological sections for microdissection genotyping. NI, Noninformative status for both gene polymorphisms.
The relatively small size of tumorlets posed a concern for allelic dropout (ADO) during PCR amplification, which may occur when nucleic acid amplification preferentially favors one allelic DNA template over the other because of limiting quantities of starting DNA. 34,35 The phenomenon was originally described in the context of single-cell PCR, but has been described in situations where genotyping is performed on biopsy samples of fixed tissue specimens. 22 The negative effect of tissue fixation on DNA integrity may not be apparent to the worker assuming that the volume of selected fixed tissue yields an equivalent amount of functional DNA templates to initiate nucleic acid amplification. To control for ADO, microdissected samples of normal tissue and carcinoid where carefully taken to be no larger in size than corresponding tumorlet targets (Figure 1) ▶ . Furthermore, the normal tissue sample was required, in informative subjects, to display polymorphic DNA sequencing bands of equivalent intensity following DNA sequencing consistent with an equal number of DNA templates undergoing sequencing (Figure 2) ▶ . As a final check, in subjects informative for both int-2 DNA sequence polymorphisms, the presence and degree of allelic imbalance had to be consistent at both polymorphic sites.
Patients were designated as informative when the normal lung sample manifested differing alleles at one or both sites of DNA sequence polymorphism (Figure 2) ▶ . To guard against allelic dropout due to small sample size and/or limiting amounts of amplifiable fixed DNA, the polymorphic bands of normal samples were required to be of approximately equal intensity, corresponding to equivalent representation and amplification of gene copies (Figure 2) ▶ . While the analysis for band intensity could have been quantified by automated densitometry, naked eye examination was found to be sufficient in all cases. Optimization of fixed specimen preparation and nucleic acid amplification was important in avoiding ADO of genotyping samples.
Ten of 26 subjects (38%) with carcinoids and/or tumorlets in this study were found to be noninformative for DNA sequence polymorphisms in intron 2 of the int-2 gene (Table 1) ▶ . The remaining 16 subjects yielded 11 carcinoids and 11 tumorlets for allelic imbalance analysis. Eight of 11 (73%) carcinoids in informative subjects demonstrated allelic imbalance (loss of heterozygosity) of the int-2 gene at 11q13. In contrast, only one of 11 tumorlets (9%) manifested allelic imbalance. The finding of int-2 allelic imbalance in informative subjects was significantly associated with carcinoid tumor formation when present (P < 0.01) and significantly associated with tumorlet formation when absent ((P < 0.01). No significant difference was present between groups with tumorlets only, carcinoid only, and carcinoid with associated tumorlets (Table 1) ▶ .
In four cases where carcinoid tumors showed loss of heterozygosity involving the int-2 gene, one or more coexistent tumorlets were seen. In no instance did the tumorlets show the same loss of heterozygosity. This finding is consistent with the concept that tumorlets in these instances did not represent intramucosal spread of carcinoid cells, but instead represented de novo independent cell proliferations. Of 11 tumorlets in informative subjects, only one showed loss of heterozygosity for the int-2 gene. This finding implied that in the spectrum of neuroendocrine proliferation, tumorlets are hyperplasias that may undergo mutational events resulting in enlargement and evolution toward bona fide carcinoid tumors. Genetic alterations in the region of 11q13 involving the int-2 gene or other closely situated genes may play an important role in this evolution.
To explore the possibility that int-2 gene allelic imbalance was uniquely associated with neuroendocrine neoplasia progression or that it was a nonspecific genetic aberration related to tumor progression, selected tumors in this series were evaluated for allelic imbalance of genes situated at 3p25 (von Hippel–Lindau), 11p13 (Wilms’ tumor), and 17p13.1 (p53). As shown in Table 2 ▶ , representative tumors with int-2 allelic imbalance did not demonstrate evidence of tumor suppressor gene loss at any of the loci studied. The findings support a causal role of 11q13 gene alteration in the pathogenesis of neuroendocrine tumor progression.
Table 2.
Int-2 (11q13.1) Allelic Imbalance in Pulmonary Carcinoid and Tumorlets
| Negative allelic imbalance | Positive allelic imbalance | Noninformative | |
|---|---|---|---|
| Pulmonary carcinoids with associated tumorlet(s) | |||
| Carcinoid | 1 | 4 | 3 |
| Tumorlet | 4 | 0 | 10 |
| Pulmonary carcinoid only | 2 | 4 | 4 |
| Pulmonary tumorlet only | 6 | 1 | 3 |
Conclusions
Pulmonary carcinoid and tumorlet share a similar histogenesis as well as many histopathological features. The two entities are, however, distinct in that the tumorlet is a benign, asymptomatic lesion not associated with neoplastic progression. Typical carcinoid tumors, while biologically benign in most instances, do possess the ability to undergo malignant progression with metastatic potential. 2 The two processes are likely related, with carcinoid tumor evolving from precursor tumorlet lesions. 1 The mechanism for this progression is not fully understood but likely involves critical genetic alterations causing progressive growth deregulation. 3
It is frequently observed that patients with carcinoid tumors may harbor one or more tumorlets, which may occur in proximity or at a distance from the carcinoid tumor. In these situations, the issue arises whether the tumorlets represent intramucosal spread of carcinoid tumor cells or independent cell proliferation. If the latter is true, a secondary issue arises: whether such subjects are experiencing a field effect for neuroendocrine cell neoplasia development.
The MEN1 gene, located at 11q13.1, is one of several candidate genes that may play a role in neuroendocrine tumor development and progression. 4,5 Point mutational change has been demonstrated in patients with familial forms of neuroendocrine neoplasia. 16-19 The gene product is believed to function as a tumor suppressor gene analogous to other well-characterized genes such as retinoblastoma (RB-1), Wilms’ tumor (WT-1), and p53. It is reasonable, therefore, to determine whether genetic damage to MEN1 may play a casual role in neoplastic progression in neuroendocrine neoplasia, with pulmonary tumorlet-carcinoid spectrum as a specific example. To accomplish this mission, two conditions must be met. First, a system of genetic analysis must be formulated to determine the presence of MEN1 gene damage. Second, the analytical approach must be adapted for use with fixative-treated tissue specimens typical of that encountered in routine surgical pathology practice.
Inasmuch as the MEN1 gene has only recently been described, and detailed sequence information including the presence and frequency of intragenic polymorphisms is not readily available, the int-2 gene, situated in proximity to MEN1 at 11q13, was chosen as a marker for allelic imbalance analysis. The int-2 gene has been studied in a variety of human cancers where it contributes to the malignant phenotype. 24,36-38 For example, in breast cancer, amplification of int-2 has been correlated with metastatic spread, local and distant tumor recurrence, and poorer overall patient survival. 38 Disturbances in int-2 have also been described in neuroendocrine tumors; however, its effects have not been dissociated from other potential genetic alterations occurring at 11q13. 39,40 This raises the possibility that other potentially important tumor suppressor genes may exist in proximity to the MEN1 gene. Mutational damage involving these genes may lead to allelic deletion detectable by loss of heterozygosity analysis of included genes such as int-2. Thus allelic imbalance analysis based on sequence polymorphisms such as that used here for the int-2 gene does not necessary implicate this gene as a critical event into tumor progression. Rather, the changes present may be used as a marker for 11q13 gene damage. The genomic sequence of int-2 has been reported and is available in molecular biology databases in the public domain. 26 Several DNA sequence polymorphisms have been described, including two in the second intron, with common frequency in the general population. 26
Tumor suppressor gene loss results in imbalance in the relative level of each allele, which may be determined by molecular approaches that define the relative content of each allele. 21 The strategies involve demonstration of loss of heterozygosity in subjects first shown to be informative for a particular marker for the gene of interest. Single base sequence polymorphisms are the most common type of polymorphism present in the human genome. 41 The method used in this study was based on such polymorphisms to characterize the relative content of each int-2 allele. Highly representative tissue was sampled by microdissection to ensure optimal exclusion of normal tissue from tumor samples (Figure 1) ▶ . Nucleic acid amplification encompassed sites of polymorphic bases that were sequenced by dideoxy chain termination. The single base difference between the two alleles did not result in any detectable changes in the efficiency of amplification or sequencing (Figure 2) ▶ . Thus a strategy of competitive PCR and sequencing could be relied on to provide accurate and reliable allelic content information. It was fortuitous that two single nucleotide polymorphisms were present sufficiently close to each other to appear in the same DNA sequencing run (Figure 2) ▶ . These two sequence polymorphisms were not linked, and so the yield of informative cases was increased because of the greater likelihood of a subject showing heterozygosity at one of the polymorphic sites.
During preliminary studies, allelic dropout (ADO) 34,35 was felt to be an important interfering factor. Unbalanced amplification of alleles can occur when limiting quantities of DNA templates form the basis for the initiation of PCR. This was first described in the context of single-cell PCR but is applicable to fixed tissue amplification of small specimens such as biopsies. 22 The actual content of amplifiable DNA templates may be far less than expected because of the negative effects of tissue fixation and processing. For this reason we optimized tissue preparation and nucleic acid amplification, using microdissection samples no larger in size than that obtained for the specific lesion in this series that yielded the smallest samples. In this series, tumorlets samples ranged in size from 1 to 3 mm, and thus all normal and carcinoid samples taken were of similar size (Table 3) ▶ . All normal tissue specimens in informative subjects required equivalent intensity of polymorphic bands on DNA sequencing to exclude the ADO effect and qualify the tumor samples for allelic imbalance genotyping.
Table 3.
Allelic Imbalance Outside 11q13 in Pulmonary Carcinoid and Tumorlets
| Negative allelic imbalance | Positive allelic imbalance | NI | |||
|---|---|---|---|---|---|
| Pulmonary carcinoids with associated tumorlet(s) | |||||
| Carcinoid | 11p13 | 2 | 11p13 | 0 | 2 |
| 3p25 | 1 | 3p25 | 0 | 1 | |
| 17p13.1 | 3 | 17p13.1 | 0 | 1 | |
| Tumorlet | 11p13 | 2 | 11p13 | 0 | 2 |
| 3p25 | 1 | 3p25 | 0 | 1 | |
| 17p13.1 | 3 | 17p13.1 | 0 | 1 | |
| Pulmonary carcinoid only | 11p13 | 2 | 11p13 | 0 | 1 |
| 3p25 | 1 | 3p25 | 0 | 2 | |
| 17p13.1 | 3 | 17p13.1 | 0 | 2 | |
| Pulmonary tumorlet only | 11p13 | 3 | 11p13 | 0 | 4 |
| 3p25 | 2 | 3p25 | 0 | 1 | |
| 17p13.1 | 4 | 17p13.1 | 0 | 3 |
NI, Noninformative status for gene polymorphism.
In this study we demonstrated a striking difference in incidence in int-2 allelic imbalance between pulmonary tumorlets and carcinoids. In subjects informative for the intragenic int-2 DNA sequence polymorphisms used in this series, eight of 11 carcinoid tumors (73%) manifested allelic imbalance. In contrast, only one of 11 tumorlets exhibited allelic imbalance (9%). Notwithstanding the small size of this series, the difference in incidence was found to be significant (P < 0.01). The data are not sufficient to causally implicate int-2 gene alterations as being directly responsible for neuroendocrine tumor progression, inasmuch as other tumor suppressor genes and/or oncogenes situated in the vicinity of int-2 at 11q13 may be primarily accountable for the allelic imbalance findings in this report. For example, there is good experimental and clinical evidence that MEN1 gene alterations may be more critical with regard to neuroendocrine cell carcinogenesis. Given the close positioning of int-2 to MEN1, it is reasonable that MEN1 gene loss may overlap the int-2 region, leading to allelic imbalance as demonstrated here. The availability of multiple DNA sequence polymorphisms, not in linkage dysequilibrium, with a high rate of heterozygosity in the general population, supports its use as a marker for 11q13 genetic alterations.
In this study we found no instance of allelic imbalance of a tumorlet occurring in association with the carcinoid tumor. This finding provides strong support against the thesis that tumorlets represent intramucosal spread of neoplastic neuroendocrine cells from carcinoid tumors. Rather, it supports the concept of independent de novo neuroendocrine cell proliferation. This conclusion is based on the fact that allelic imbalance at 11q13 is likely to confer a proliferative advantage for clonal expansion and thus would not likely give rise to invasive tumor cells lacking this property once acquired. In a similar fashion, the findings reported herein raise the possibility that multifocal carcinoid tumors, an event which while infrequent has been noted to occur, may represent development and progression of independent primary tumors. No case of multifocal carcinoid tumors was present in this study.
Acquisition of int-2 allelic imbalance would appear to be an important early marker for neoplastic progression in neuroendocrine cell neoplasia. As such intermediate and high grades of neuroendocrine malignancy, including atypical carcinoid and small and large cell neuroendocrine carcinoma, may be expected to manifest a similar high frequency of allelic imbalance. A search of the current literature provides support in this regard. 42,43 Unfortunately, this tumor is well known for extensive genetic alterations, of which 11q damage may represent a later epiphenomenon. 11q13 need not be an obligate event, because progression to high-grade malignancy may involve a unique set of genetic alterations independent of those causally related to biologically less aggressive forms of tumor development.
Finally, we wish to bring attention to the methodological approach used in this study to determine allelic imbalance on minute, microdissected tissue samples. Tumor suppressor gene loss can be accomplished in a variety of ways, including competitive PCR and restriction endonuclease (RE) digestion. The former is not practical, because it is difficult to ensure equivalence in nucleic acid amplification of separate genes, each with its own unique oligonucleotide primer sets. RE digestion is based on principles similar to those used here, being formatted on the presence in informative subjects of DNA sequence difference between two alleles. Our approach, which uses comparative DNA sequencing band intensity, is not limited by the availability of RE sites at points of DNA polymorphisms. Potential differences in PCR amplification are controlled in that the template sequence for amplification is identical, save for the point(s) of sequence polymorphism, and comparison is made with a control taken at approximately the same size from tissue subject to identical fixation conditions. Given the rapidly expanding database of human DNA sequence information, we recommend our approach as a means of evaluating the role of gene content alterations in all types and sizes of clinical specimens.
Footnotes
Address reprint requests to Dr. Sydney D. Finkelstein, Department of Pathology, UPMC-Presbyterian A610.2, 200 Lothrop St., Pittsburgh, PA 15213. E-mail: finkelsteinsd@msx.upmc.edu.
References
- 1.Vuitch F, Sekido Y, Fong K, Mackay B, Minna JD, Gazdar AF: Neuroendocrine tumors of the lung. Pathology and molecular biology. Chest Surg Clin North Am 1997, 7:21-47 [PubMed] [Google Scholar]
- 2.Marchevsky AM: Neuroendocrine tumors of the lung. Pathology 1996, 4:103-123 [PubMed] [Google Scholar]
- 3.Pearson PL, Van der Luijt RB: The genetic analysis of cancer. J Intern Med 1998, 243:413-417 [DOI] [PubMed] [Google Scholar]
- 4.Chandrasekharappa SC, Guru SC, Manickam P, Olufemi SE, Collins FS, Emmert-Buck MR, Debelenko LV, Zhuang Z, Lubensky IA, Liotta LA, Crabtree JS, Wang Y, Roe BA, Weisemann J, Boguski M, Agarwal SK, Kester MB, Kim YS, Heppner C, Dong Q, Speigel AM, Burns AL, Marx SJ: Positional cloning of the gene for multiple endocrine neoplasia—Type 1. Science 1997, 276:404-406 [DOI] [PubMed] [Google Scholar]
- 5.Lemmens I, Van de Ven WJM, Kas Koen, Zhang CX, Giraud S, Wautot V, Buisson N, Witte KD, Salabdre J, Lenoir G, Pugeat M, Calender A, Parente F, Quincey D, Gaudray P, Wit MJD, Lips CJM, Hoppener JWM, Khodaei S, Grant AL, Weber G, Kytola S, The BT, Farnebo F, Phelan C, Haywood N, Larsson C, Pannett AAJ, Forbes SA, Bassett JHD, Thakker RV: Identification of the multiple endocrine neoplasia type 1 (MEN1) gene. Hum Mol Genet 1997, 6:1177–1183 [DOI] [PubMed]
- 6.Bale AE, Norton JA, Wong EL, Fryburg JS, Maton PN, Oldfield EH, Streeten E, Aurbach GD, Brandi ML, Friedman E, Spiegel AM, Taggart RT, Marx SJ: Allelic loss on chromosome 11 in hereditary and sporadic tumors related to familial multiple endocrine neoplasia type 1. Cancer Res 1991, 51:1154-1157 [PubMed] [Google Scholar]
- 7.Eng C: The RET proto-oncogene in multiple endocrine neoplasia type 2, and Hirschsprung’s disease in Seminars in Medicine of the Beth Israel Hospital. New Engl J Med 1996, 335:943-951 [DOI] [PubMed] [Google Scholar]
- 8.Anonymous: The search for the MEN1 gene. The European Consortium on MEN-1. J Intern Med 1998, 243:441–446 [DOI] [PubMed]
- 9.Kovatich A, Friedland DM, Druck T, Hadeczek P, Huebner K, Comis RL, Hauck W, McCue PA: Molecular alterations to human chromosome 3p loci in neuroendocrine lung tumors. Cancer 1998, 83:1109-1117 [DOI] [PubMed] [Google Scholar]
- 10.Cagle PT, El-Naggar AK, Xu H-J, Hu S-X, Benedict WF: Differential retinoblastoma protein expression in neuroendocrine tumors of the lung. Am J Pathol 1997, 150:393-400 [PMC free article] [PubMed] [Google Scholar]
- 11.Gouyer V, Gazzeri S, Brambilla E, Bolon I, Moro D, Perron P, Benabid AL, Brambilla C: Loss of heterozygosity at the RB locus correlates with loss of RB protein in primary malignant neuro-endocrine lung carcinomas. Int J Cancer 1994, 58:818-824 [DOI] [PubMed] [Google Scholar]
- 12.Brambilla E, Negoescu A, Gazzeri S, Lantuejoul S, Moro D, Brambilla C, Coll JL: Apoptosis-related factors p53, Bcl2, and bax in neuroendocrine lung tumors. Am J Pathol 1996, 149:1941-1952 [PMC free article] [PubMed] [Google Scholar]
- 13.Roncalli M, Doglioni C, Springall DR, Papotti M, Pagani A, Polak JM, Ibrahim NBN, Coggi G, Viale G: Abnormal p53 expression in lung neuroendocrine tumors. Diagn Mol Pathol 1992, 1:129-135 [PubMed] [Google Scholar]
- 14.Hiyama K, Hasegawa K, Ishioka S, Takahashi N, Yamakido M: An atypical carcinoid tumor of the lung with mutations in the p53 gene and the retinoblastoma gene. Chest 1993, 104:1606-1607 [DOI] [PubMed] [Google Scholar]
- 15.Przygodzki RM, Finkelstein SD, Langer JC, Swalsky PA, Fishback N, Bakker A, Guinee DG, Koss M, Travis WD: Analysis of p53, K-ras-2, and C-raf-1 in pulmonary neuroendocrine tumors. Am J Pathol 1996, 148:1531-1541 [PMC free article] [PubMed] [Google Scholar]
- 16.Agarwal SK, Kester MB, Debelenko LV, Heppner C, Emmert-Buck MR, Skarulis MC, Doppman JL, Kim YS, Lubensky IA, Zhuang Z, Green JS, Guru SC, Manickam P, Olufemi SE, Liotta LA, Chandrasekharappa SC, Collins FS, Spiegel AM, Burns AL, Marx SJ: Germline mutations of the MEN1 gene in familial multiple endocrine neoplasia type 1 and related states. Hum Mol Genet 1997, 6:1169-1175 [DOI] [PubMed] [Google Scholar]
- 17.Bassett JHD, Forbes SA, Pannett AAJ, Lloyd SE, Christie PT, Wooding C, Harding B, Besser GM, Edwards CR, Monson JP, Sampson J, Wass JAH, Wheeler MH, Thakker RV: Characterization of mutations in patients with multiple endocrine neoplasia Type 1. Am J Hum Genet 1998, 62:232-244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Debelenko LV, Emmert-Buck MR, Zhuang Z, Epshteyn E, Moskaluk CA, Jensen RT, Liotta LA, Lubensky IA: The multiple endocrine neoplasia type 1 gene locus is involved in the pathogenesis of type II gastric carcinoids. Gastroenterology 1997, 113:773-781 [DOI] [PubMed] [Google Scholar]
- 19.Debelenko LV, Brambilla E, Agarwal SK, Swalwell JI, Kester MB, Lubensky IA, Zhuang Z, Guru S, Manickam P, Olufemi SE, Chandrasekharappa SC, Crabtree JS, Kim YS, Heppner C, Burns AL, Spiegel AM, Marx SJ, Liotta LA, Collins FS, Travis WD, Emmert-Buck MR: Identification of MEN1 gene mutations in sporadic carcinoid tumors of the lung. Hum Mol Genet 1997, 6:2285-2290 [DOI] [PubMed] [Google Scholar]
- 20.Knudson AG: Antioncogenes and human cancer. Proc Natl Acad Sci USA 1993, 90:10914-10921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elias JM: Introduction ot the genetics of cancer. Cell Vision 1998, 5:37-42 [PubMed] [Google Scholar]
- 22.Finkelstein SD, Swalsky PA, Przygodzki RM: Microdissection-based p53 genotyping: concepts for molecular testing. Mol Diagn 1998, 3:179-191 [DOI] [PubMed] [Google Scholar]
- 23.Rao U, Swalsky PA, Bakker A, Finkelstein SD: Desmoplastic melanoma. Mod Pathol 1999, 12:344-350 [PubMed] [Google Scholar]
- 24.Yoshida MC, Wada M, Satoh H, Yoshida T: Human HST1 (HSTF1) gene maps to chromosome band 11q13, and coamplifies with the INT2 gene in human cancer. Proc Natl Acad Sci USA 1988, 85:4861-4864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Polymeropoulos MH, Xiao H, Rath DS, Merril CR: Dinucleotide repeat polymorphism at the int-2 proto-oncogene locus (INT2). Nucleic Acids Res 1990, 18:7468. [PMC free article] [PubMed] [Google Scholar]
- 26.Meyers SL, Dudley JP: Sequence analysis of the int-2/fgf-3 gene in aggressive human breast carcinomas. Mol Carcinog 1992, 6:243-251 [DOI] [PubMed] [Google Scholar]
- 27.Finkelstein SD, Sayegh R, Christensen S, Swalsky PA: Genotypic classification of colorectal adenocarcinoma. Cancer 1993, 71:3827-3838 [DOI] [PubMed] [Google Scholar]
- 28.Finkelstein SD, Przygodzki RM, Pricolo VE, Sakallah SA, Swalsky PA, Bakker A, Lanning R, Bland KI, Cooper DL: Prediction of biological aggressiveness in colorectal cancer by p53/k-ras-2 topographic genotyping. Mol Diagn 1996, 1:5-28 [DOI] [PubMed] [Google Scholar]
- 29.Pollack IF, Hamilton RL, Finkelstein SD, Campbell JW, Martinez AJ, Sherwin RN, Bozik ME, Gollin SM: The relationship between mutations and overexpression of p53 and prognosis in malignant gliomas of childhood. Cancer Res 1997, 57:304-309 [PubMed] [Google Scholar]
- 30.Kounelis S, Jones MW, Papadaki H, Bakker A, Swalsky PA, Finkelstein SD: Carcinosarcomas (malignant mixed mullerian tumors) of the female genital tract: comparative molecular analysis of epithelial and mesenchymal components. Hum Pathol 1998, 29:82-87 [DOI] [PubMed] [Google Scholar]
- 31.Gnarra JR, Tory K, Weng Y, Schmidt L, Wei MH, Li H, Latif F, Liu S, Chen F, Duh FM, Lubensky IA, Duan DR, Florence C, Pozzatti R, Walther MM, Bander NH, Grossman HB, Brauch H, Pomer S, Brooks JD, Isaacs WB, Lerman MI, Zbar B, Linehan WM: Mutations of the VHL tumor suppressor gene in renal carcinoma. Nature Genet 1994, 7:85-90 [DOI] [PubMed] [Google Scholar]
- 32.Haber DA, Buckler AJ, Glaser T, Call KM, Pelletier J, Sohn RL, Douglass EC, Housman DE: An internal deletion within an 11p13 zinc finger gene contributes to the development of Wilms’ tumor. Cell 1990, 61:1257-1269 [DOI] [PubMed] [Google Scholar]
- 33.Hahn M, Fislage R, Pingoud A: Polymorphism of the pentanucleotide repeat d(AAAAT) within intron 1 of the human tumor suppressor gene p53 (17p13.1). Hum Genet 1994, 93:222. [DOI] [PubMed] [Google Scholar]
- 34.Dressen JC, Bras M, Coonen E, Dumoulin JC, Evers JL, Geraedts JP: Allelic dropout caused by allele-specific amplification failure in single-cell PCR of the cystic fibrosis delta F508 deletion. J Assist Reprod Genet 1996, 13:112-114 [DOI] [PubMed] [Google Scholar]
- 35.Rechitsky S, Freidine M, Verlinsky Y, Strom CM: Allele dropout in sequential PCR and FISH analysis of single cells (cell recycling). J Assist Reprod Genet 1996, 13:115-124 [DOI] [PubMed] [Google Scholar]
- 36.Lese CM, Rossie KM, Appel BN, Reddy JK, Johnson J, Myers EN, Gollin SM: Visualization of INT2 and HST1 amplification in oral squamous cell carcinomas. Genes Chromosom Cancer 1995, 12:288-295 [DOI] [PubMed] [Google Scholar]
- 37.Bale SJ, Bale AE, Stewart K, Dachowski L: Lingake analysis of multiple endocrine neoplasia type 1 with INT2 and other markers on chromosome 11. Genomics 1989, 4:320-322 [DOI] [PubMed] [Google Scholar]
- 38.Pauley RJ, Gimotty PA, Paine TJ, Dawson PJ: INT2 and ERBB2 amplification and ERBB2 expression in breast tumors from patients with different outcomes. Breast Cancer Res Treat 1996, 37:65-76 [DOI] [PubMed] [Google Scholar]
- 39.Debelenko LV, Zhuang, Z, Emmert-Buck MR, Settara C, Chandrasekharappa SC, Manickam P, Guru SC, Marx SJ, Skarulis MC, Spiegel AM, Collins FS, Jensen RT, Liotta LA, Lubinsky IA: Allelic deletions on chromosome 11q13 in multiple endocrine neoplasia type 1-associated and sporadic gastrinomas and pancreatic endocrine tumors. Cancer Res 1997, 57:2238–2243 [PubMed]
- 40.Dong Q, Debelenko LV, Chandrasekharappa SC, Emmert-Buck MR, Guru SC, Manickam P, Olufemi SE, Skarulis MC, Doppman JL, Alexander RH, Kim YS, Saggar SK, Lubensky IA, Zhuang Z, Liotta LA, Chandrasekharappa SC, Collins FS. Spiegel AM, Burns AL, Marx SJ: Loss of heterozygosity at 11q13: analysis of pituitary tumors, lung carcinoids, lipomas, and other uncommon tumors in subjects with familial multiple endocrine neoplasia type 1. J Clin Endocrinol Metab 1997, 82:1416–1420 [DOI] [PubMed]
- 41.Landegren U, Nilsson M, Kwok PY: Reading bits of genetic information: methods for single nucleotide polymorphism analysis. Genome Res 1998, 8:769-776 [DOI] [PubMed] [Google Scholar]
- 42.Walch AK, Zitzelsberger HF, Aubele MM, Mattis AE, Bauchinger M, Candidus S, Prauer HW, Werner M, Hofler H: Typical and atypical carcinoid tumors of the lung are characterized by 11q deletions as detected by comparative genomic hybridization. Am J Pathol 1998, 153:1089-1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Onuki N, Wistuba II, Travis WD, Virmani AK, Yashima K, Brambilla E, Hasleton P, Gazdar AF: Genetic changes in the spectrum of neuroendocrine lung tumors. Cancer 1999, 85:600-607 [DOI] [PubMed] [Google Scholar]