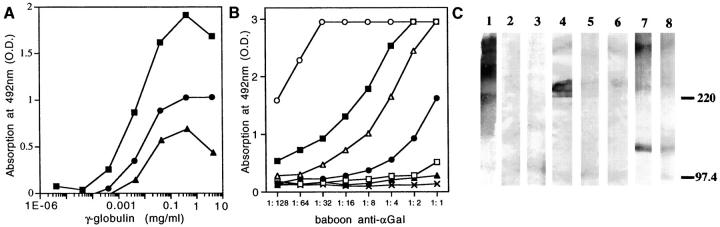
Figure 2.
Reactivity of baboon anti-αGal and baboon anti-PAEC with the extracellular matrix. A: ELISA reactivity of baboon anti-αGal, baboon anti-PAEC, and pre-immune baboon γ-globulins with Matrigel. Binding of baboon anti-αGal (▪) was 328-fold, and that of baboon anti-PAEC (•) 19-fold higher than that of pre-immune baboon γ-globulin (▴), (P < 0.0001) B: Binding of baboon anti-αGal to αGal/BSA, pig thyroglobulin, and to components of the extracellular matrix, measured by ELISA. The highest binding was for mouse laminin (▪), followed by porcine thyroglobulin (▵), mouse HSPG (•), and bovine fibronectin (□). The antibody did not react with the nephritogenic tubular basement membrane antigen (▴) and with human laminin (X), or with bovine chondroitin sulfate, hyaluronate, bovine collagen I, II, and III, pig collagen I and II, and BSA (not shown). (○) αGal/BSA. C: Western blot analysis of extracellular matrix components probed with 10 μg/ml baboon anti-αGal IgG. This antibody recognized epitopes expressed on mouse laminin (lane 1), bovine fibronectin (lane 4), and mouse HSPG (lane 7), not epitopes on human laminin (lane 3), and human fibronectin (lane 6). Absorption with rabbit erythrocytes abolished reactivity with laminin (lane 2), bovine fibronectin (lane 5) and, partially, mouse HSPG (lane 8).