Figure 1.
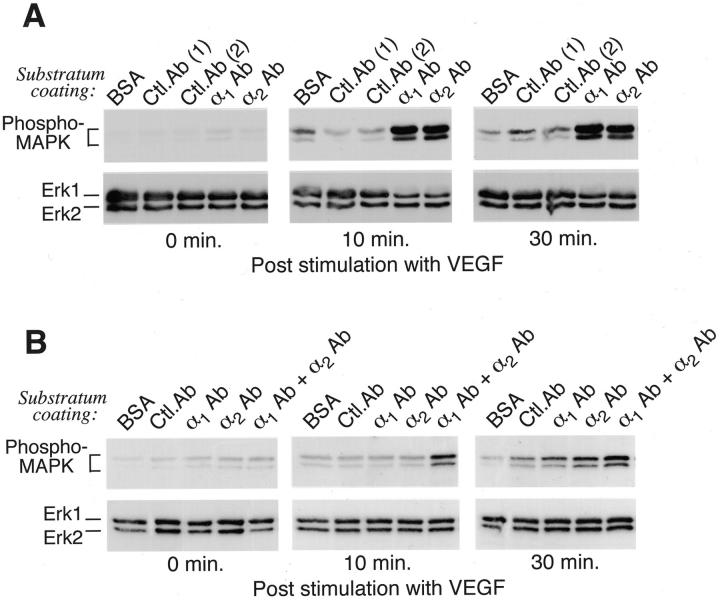
Adhesion of dermal microvascular ECs through either the α1β1 integrin or the α2β1 integrin supports VEGF activation of Erk1/Erk2 MAP kinases. Functional integrin Abs or control Abs were immobilized on plastic and remaining protein-binding sites were blocked with BSA, as described in Materials and Methods. After plating, cells attached and spread on substratum coated with α1 Ab or α2 Ab, but remained unattached to wells coated with isotype control Abs or BSA alone. After plating, MAPK phosphorylation was allowed to decay for 3 hours before stimulation with VEGF. A: Wells coated with 10 μg/ml Ab. As indicated by staining with phospho-specific MAPK Abs, VEGF induced marked activation in cells plated on α1 Ab or α2 Ab but poorly induced MAPK activation in cells plated on control Abs or BSA. Staining with Ab that recognizes both phosphorylated and nonphosphorylated forms of Erk1 and Erk2 established that they were present equally in all samples. B: VEGF more efficiently induced activation of MAPK in cells plated on substratum coated with a combination of α1 Ab and α2 Ab (0.2 μg/ml of each) than in cells plated on substratum coated with each Ab alone (0.2 μg/ml). For all experiments (A and B), failure of VEGF to activate MAPK in cells plated on BSA or control (Ctl) Abs was not attributable to anoikis because cell viability remained >90%, as determined by replating of cells on collagen-coated plastic.