Abstract
Abnormal uterine bleeding is the leading indication for discontinuation of long-term progestin-only contraceptives (LTPOCs). Histological sections of endometria from LTPOC-treated patients display abnormally enlarged blood vessels at bleeding sites. Paradoxically, a trend toward reduced endometrial perfusion in LTPOC users has been reported in these patients. We hypothesized that hypoxia/reperfusion-induced free radical production inhibits the expression of angiopoietin-1 (Ang-1), a vessel stabilizing factor, leaving unopposed the effects of endothelial Ang-2, a vessel-branching and permeability factor. Immunohistochemical studies confirmed selective decreases in stromal cell Ang-1 in LTPOC-exposed endometrium. To indirectly assess whether LTPOC enhances endometrial free radical production, immunostaining was conducted for the phosphorylated form of the stress-activated kinases SAPK/JNK and p38. These kinases were greatly increased in endometria from LTPOC-treated patients. Interestingly, the endothelial cells but not the stromal cells displayed enhanced immunostaining for the phosphorylated mitogen-activated kinase (pMAPK) after LTPOC treatment. To further examine the effects of progestin, hypoxia, and reactive oxygen species (ROS) on the regulation of Ang-1 and Ang-2 as well as the activation of MAPK, SAPK/JNK, and p38 by the relevant cell types, we conducted in vitro studies with cultured human endometrial stromal cells (HESCs) and human endometrial endothelial cells (HEECs). Cultures of HESCs were treated with vehicle control, estradiol (E2), or with medroxyprogesterone acetate ± E2 under hypoxic and normoxic conditions. Although medroxyprogesterone acetate but not E2 increased Ang-1 expression, hypoxia greatly decreased Ang-1 protein and mRNA expression. In contrast, HESCs did not appear to express Ang-2 protein or mRNA. Conversely, cultured HEECs did not appear to express Ang-1, but expressed Ang-2, the levels of which were significantly increased by hypoxia. Hypoxia also induced the phosphorylation of SAPK/JNK and p38 in both cultured HESCs and HEECs. Moreover, ROS such as that observed after hypoxia/reperfusion resulted in the activation of SAPK/JNK and p38 in HESCs and HEECs and inhibited Ang-1 in cultured HESCs. These effects could be blocked by oxygen radical scavengers. Consistent with the in vivo studies, MAPK was activated after ROS treatment in HEECs but not in HESCs. Our findings suggest that LTPOC-induced endometrial bleeding occurs as a result of hypoxia/reperfusion-induced free radicals that directly damage vessels and alter the balance of Ang-1 and Ang-2 to produce the characteristic enlarged and permeable vessels that are prone to bleeding.
Long-term progestin-only contraceptives (LTPOC) provide a highly practical solution to family-planning needs in developed and developing countries. Unfortunately, bleeding in the early months of treatment often results in the discontinuation of these otherwise safe and efficacious contraceptive methods. Several studies have shown that levonorgestrel (LNG) administered either locally to the uterine cavity or systemically results in very significant morphological changes in the endometrium including gland atrophy, extensive decidual transformation of the stroma, and increased endometrial vasculature density. 1-3 With long-term LNG contraception, the vessels are enlarged, dilated, thin-walled, and fragile, suggesting they are the product of aberrant angiogenesis. 4,5 Indeed, a number of investigators have found a link between LTPOC-associated bleeding and aberrant expression of the angiogenic agent vascular endothelial growth factor (VEGF). 6-8 Immunostaining for VEGF in endometrial glands and stroma was significantly increased after LTPOC therapy, however, no correlation was found between the VEGF-staining index and endometrial microvascular density. 7
Although VEGF initiates angiogenesis, it is now known that another family of proteins, the angiopoietins acting via the Tie-2 receptor, are key regulators of the subsequent angiogenic steps including vessel branching, maturation, and stabilization. Angiopoietin-1 (Ang-1) promotes vascular integrity by optimizing the integration of the endothelial cells with the surrounding supporting cells. Recently it was reported that Ang-1 protects the adult vasculature from bleeding by countering the permeability effects observed after excess exposure to VEGF. 9,10 In contrast, Ang-2 is a partial antagonist of Ang-1/Tie-2 interactions and is generally expressed in areas undergoing vascular remodeling. 11 Like VEGF, Ang-2 enhances vascular permeability and branching. Both hypoxia and VEGF up-regulate Ang-2 expression in bovine microvascular endothelial cells. 12,13 Thus, Ang-1 and Ang-2 have complementary roles in vascular development and maintenance. Therefore, it is plausible that altered regulation of Ang-1 and Ang-2 is responsible for the pathological endometrial angiogenesis observed after LTPOC treatment.
In a recent pilot study, Hickey and colleagues 14 demonstrated a trend toward reduced endometrial perfusion in long-term progestrogen users. We hypothesized that this trend could reflect changes in the autoregulation of endometrial blood flow that likely induce focal areas of hypoxia. Recently, our laboratory has shown that Ang-2 is expressed by endometrial endothelial cells and it is strongly up-regulated by hypoxia and the inflammatory agent phorbol myristate acetate. 15 In addition, a number of investigators have demonstrated that hypoxia also up-regulates VEGF expression in endometrial stromal cells and glands. 16,17 Hypoxia is frequently followed by reperfusion and results in oxidative damage in the surrounding tissues. 18-20 Such oxidative stress has been shown to induce SAPK/JNK and p38 kinases in a number of different cell types. 21-24 In addition, increased circulating levels of lipid peroxides with concomitant depression of the antioxidant vitamin E have been reported in users of LTPOCs experiencing breakthrough bleeding. 25 Thus, we posit that LTPOC-induced hypoxia/reperfusion and/or oxidative stress promotes the dysregulation of Ang-1 and Ang-2 and gene activation resulting from the phosphorylation of stress-activated kinases in endometrial stromal and endothelial cells leading to fragile vessel formation and ultimately breakthrough bleeding. The aim of this study was to test this hypothesis by evaluating the effect of progestins, hypoxia, and oxidative stress on the expression of the angiopoietins, MAPK, SAPK/JNK, and p38 kinases in vivo and in vitro.
Materials and Methods
Tissues
Endometrial specimens for immunohistochemical studies were obtained from women with normal menstrual cycles before and 3 months after starting LTPOC with either Norplant implants (n = 6) or a LNG-releasing intrauterine system (LNG-IUS; n = 10). Pretreatment samples were obtained from either the proliferative or secretory phases of the cycle by blind pipelle biopsy (Unimar, Willon, CT). In addition, endometrial specimens were obtained from patients undergoing hysterectomies for myomas, and transported on ice to a sterile laminar flow hood for endometrial cell isolation and culture. Small portions were formalin-fixed for dating by the histological criteria of Noyes and colleagues. 26 The use of endometrial tissue for study was approved by the respective centers’ Institutional and Ethical Review Boards and written informed consent was obtained before endometrial sampling.
Immunohistochemistry
Endometrial specimens from control and LTPOC-treated patients were fixed in 4% paraformaldehyde and embedded in paraffin. Peroxidase staining was conducted with the ABC elite kit from Vector Laboratories (Burlingame, CA) as described. 15 Goat polyclonal antibodies to Ang-2 were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit polyclonal antibody to Ang-1 was obtained from Alpha Diagnostic (San Antonio, TX). Rabbit polyclonal antibodies to phosphorylated SAPK/JNK, p38, and to total or phosphorylated MAPK were obtained from Cell Signaling Technology (Beverly, MA).
Preparation of Primary Endometrial Cell Cultures
Human Endometrial Stromal Cells (HESCs)
HESCs were isolated and grown to confluence in a 37°C incubator using a basal medium as previously described. 27 For hormone treatment of HESCs, the experimental period was initiated by the addition of vehicle control or 10−8 mol/L estradiol (E2) ± 10−7 mol/L medroxyprogesterone acetate (MPA). The cells were treated for 10 days, changing the medium every 3 to 4 days. Cultures were then exposed to either normoxic or hypoxic conditions for an additional 48 hours in a serum-free defined medium as previously described. 27 The latter was achieved by placing select cultures in sealed chambers containing a portable gas oxygen analyzer and a beaker of water to maintain humidity. The chambers were then purged with 5% CO2 and 95% N2 until the oxygen analyzers read 0 to 1% O2 (12 to 14 mm Hg) and returned to the incubator for 48 hours. No changes in O2 levels were observed throughout the duration of the incubations. Experimental incubations were terminated by removing the conditioned media and extracting the cell lysate directly with either sodium dodecyl sulfate gel-loading buffer for protein studies or with Tri-reagent (Sigma, St. Louis, MO) for total RNA as previously described. 28
HEECs
HEECs were isolated and grown to confluence on flasks coated with attachment factor (Cell Systems, Kirkland, WA) in CS-C Complete Medium supplemented with 15% stripped fetal calf serum as described. 29 The cells were harvested by trypsin/ethylenediaminetetraacetic acid (EDTA) and split 1:6 for four to six passages. The experimental conditions were performed in a serum-free defined medium as described. 29 Select cultures were treated with hypoxia and harvested for protein or total RNA in the same manner as that described for the HESCs (see above).
Reactive Oxygen Species (ROS) Generation
ROS were generated either by the addition of H2O2 (50 μmol/L), FeCl2 (50 μmol/L), or both for the indicated times in defined media. Desferrioxamine (250 μmol/L), or dimethyl sulfoxide (DMSO) (28 mmol/L) were used to block oxygen radical production. All reagents for ROS were obtained from Sigma-Aldrich (St. Louis, MO).
Western Blotting
Western blotting was conducted on 5 to 15% gradient gels (Bio-Rad, Hercules, CA) as previously described. 28 Antibodies to phosphorylated SAPK/JNK, p38, and total or phosphorylated MAPK were obtained from Cell Signaling Technology. Antibodies to Ang-1 were from Alpha Diagnostic and to Ang-2 from Santa Cruz Biotechnology.
Northern Blotting
Northern blots for Ang-1 and Ang-2 were conducted as previously described. 15
Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
RT-PCR for Ang-1 and Ang-2 was conducted as follows: reverse transcription, first strand synthesis was performed with Superscript First Strand Synthesis Systems for RT-PCR (Life Technologies, Inc., Grand Island, NY) using 1 to 5 μg of total RNA and oligo(dT) (0.5 μg/μl) as recommended by the manufacturer. The reaction was terminated by incubating at 70°C for 15 minutes and followed by treatment with RNase H for 20 minutes before proceeding to the PCR step. PCR was then conducted with Platinum PCR SuperMIX (Life Technologies, Inc.) and 5 μl of template DNA derived from the first strand synthesis plus 200 mmol/L of sense and antisense primers for Ang-1 or Ang-2. The PCR primer pair for Ang-1 used is commercially available from R&D Systems (Minneapolis, MN). The product yields a fragment 582 bp long. The primer pair for Ang-2 was synthesized by Life Technologies, Inc. using the sequence published by Kim and colleagues. 30 The product yields a fragment 1535 bp long. Thirty-five cycles were performed for the PCR reaction.
RT-PCR for Tie-2 and GAPDH. Reverse transcription was conducted in the same manner as that for Ang-1 and Ang-2. The PCR step was conducted with the MPCR Kit from Maxim Biotech Inc. (San Francisco, CA) that contains primers for Tie-2 and GAPDH according to the manufacturer’s instructions. The PCR product for Tie-2 results in a 212-bp fragment and the product for GAPDH results in a 750-bp fragment.
Results
Immunohistochemical Studies
Ang-1 and Ang-2
Fixed endometrial specimens obtained before and after 3 months of LTPOC treatment were stained for Ang-1 and Ang-2. Figure 1 ▶ indicates that both secretory and proliferative endometrium stained for Ang-1 in an even manner. The highest degree of staining occurred in the endothelium with low-level staining in the stroma and in the glands. In contrast, high-dose local intrauterine progestin (LNG-IUS)-exposed endometrium displayed patchy staining for Ang-1 with some areas of high staining and some areas of low to no staining. The percentage of high versus low staining is ∼80% versus 20% (GK). In contrast to that observed in cycling endometrium, most of the staining for Ang-1 after LTPOC was observed in the stromal cells (Figure 1) ▶ . Unlike results observed with Ang-1, immunostaining for Ang-2 was mainly confined to endothelial cells in both control and LTPOC-exposed endometria (Figure 2) ▶ . Because of the high levels of Ang-2 expressed in normal cycling endometrium as well as that expressed after LTPOC, it was difficult to ascertain whether LTPOC resulted in a significant increase in endothelial cell Ang-2 expression in the endometria of LNG-treated patients compared to controls.
Figure 1.
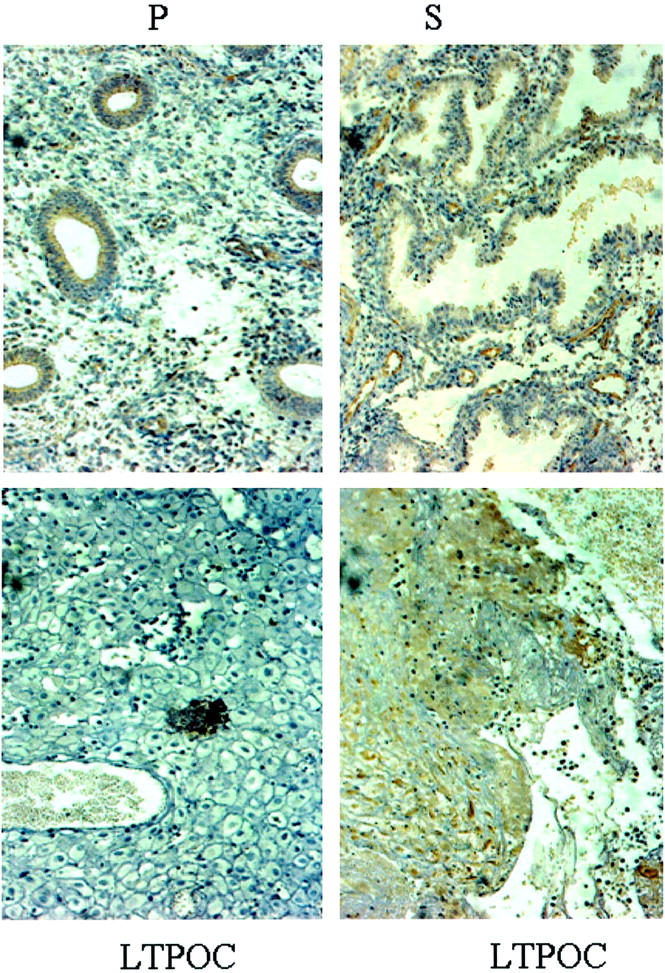
Immunohistochemistry of Ang-1 in control and LTPOC-treated human endometrium. Immunohistochemical staining for Ang-1 was performed in human endometrium from proliferative phase (P), secretory phase (S), and endometrium exposed to high-dose local intrauterine LNG (LTPOC) for 3 months. Two different areas from the same pseudo-decidualized tissue show the consistent blotchy staining pattern after LTPOC treatment reflected by an area of low to no staining (bottom left) and an area of high staining (bottom right). Studies were performed in paraffin-fixed sections as described in Materials and Methods. Antigens are identified by brown peroxidase staining. No staining was observed in the absence of primary antibody (not shown).
Figure 2.
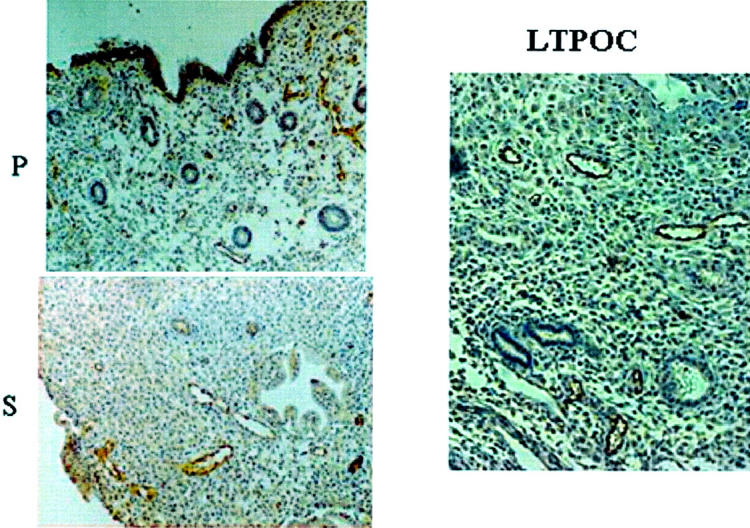
Immunohistochemistry of Ang-2 in control and LTPOC-treated human endometrium. Immunohistochemical staining for Ang-2 in human endometrium from proliferative phase (P), secretory phase (S), and LTPOC-exposed endometrium that in this case consisted of 3 months Norplant treatment. Studies were performed in paraffin-fixed sections as described in Materials and Methods. Antigens are identified by brown peroxidase staining. No staining was observed in the absence of primary antibody (not shown).
SAPK/JNK and p38
To assess whether stress pathways were activated after LTPOC treatment, sections were stained for phosphorylated SAPK/JNK and p38. As can be seen in Figure 3 ▶ , both p38 and SAPK/JNK were greatly activated after LTPOC treatment in the stromal, endothelial, and glandular epithelial cells (n = 5). MAPK was activated specifically in the endothelial cells after LTPOC. In contrast, there was no difference in immunostaining for total MAPK in LTPOC-treated versus controls (not shown).
Figure 3.
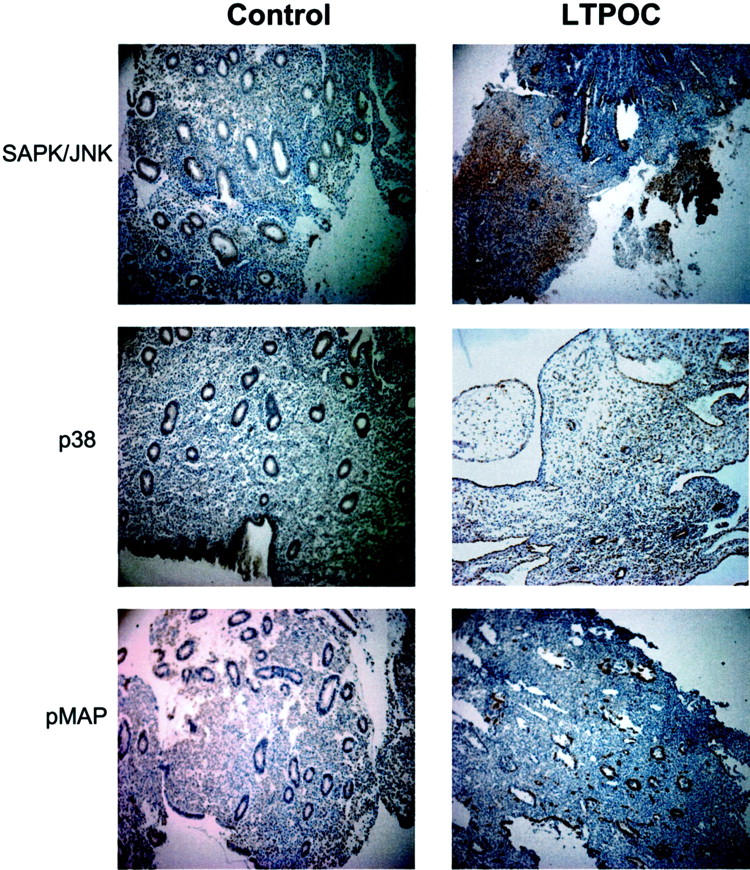
Immunohistochemistry of SAPK/JNK, p38, and pMAPK in control and LTPOC-treated human endometrium. Immunohistochemical staining for SAPK/JNK, p38, and pMAPK was conducted in human endometrium from control (proliferative phase) and endometrium exposed to high-dose intrauterine LNG (LTPOC) for 3 months. Studies were performed in paraffin-fixed sections as described in Materials and Methods. Antigens are identified by brown peroxidase staining. No staining was observed in the absence of primary antibody (not shown).
Effects of Progestin, Hypoxia, and Oxidative Stress on the Expression of Ang-1 and Ang-2 in Cultured HESCs
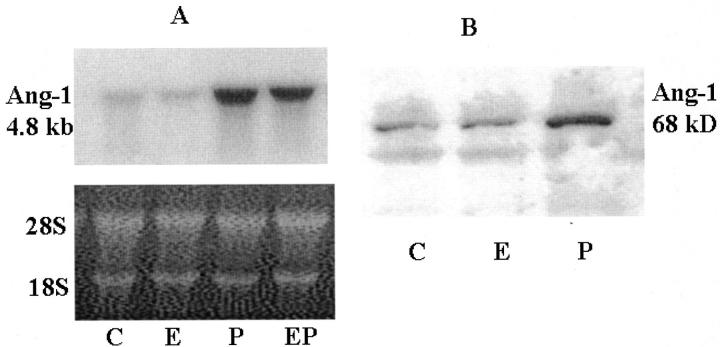
As can be seen in Figure 4A ▶ , MPA greatly enhanced the expression of Ang-1 mRNA by cultured HESCs compared to those treated with vehicle control or E2. The effects of progestin were similar in the presence or absence of E2. The expression of Ang-1 protein mimicked the results observed for mRNA (Figure 4B ▶ , Western blot).
Figure 4.
Northern and Western blots for Ang-1 in hormone-treated HESCs. HESCs were treated for 10 days with control (C), E2 (E), MPA (P), or E2 + MPA (EP) as described in Materials and Methods. A: Northern blot of HESC-expressed Ang-1 mRNA, which was detected as described in Materials and Methods. Loading efficiencies were assessed by ethidium bromide staining of the 28S and 18S ribosomal RNA. B: Western blot of HESC-expressed Ang-1 protein was conducted as described in Materials and Methods.
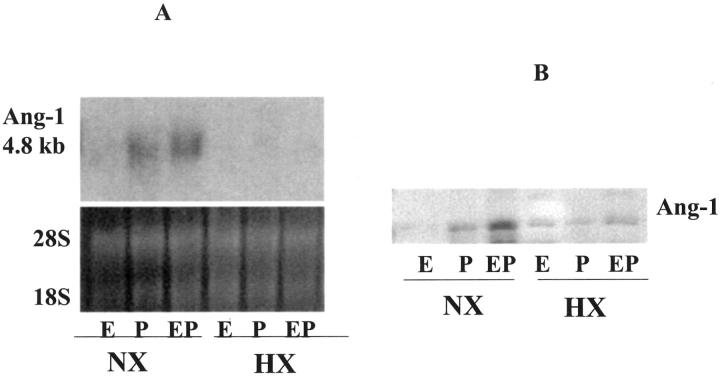
We next studied the effects of hypoxia after steroid hormone treatment of cultured HESCs. As can be seen in Figure 5, A and B ▶ , hypoxia abolished the MPA-induced expression of Ang-1 mRNA and protein expression.
Figure 5.
Northern and Western blots for Ang-1 in hormone-treated HESCs under NX and HX. HESCs were treated for 10 days with control (C), E2 (E), MPA (P), or E2 + MPA (EP). At the end of this period, they were treated for a further 48 hours under NX or HX in the presence of the appropriate hormones as described in Materials and Methods. A: Northern blot of HESC-expressed Ang-1. Loading efficiencies were assessed by ethidium bromide staining of the 28S and 18S ribosomal RNA. B: Western blot of HESC-expressed Ang-1 protein.
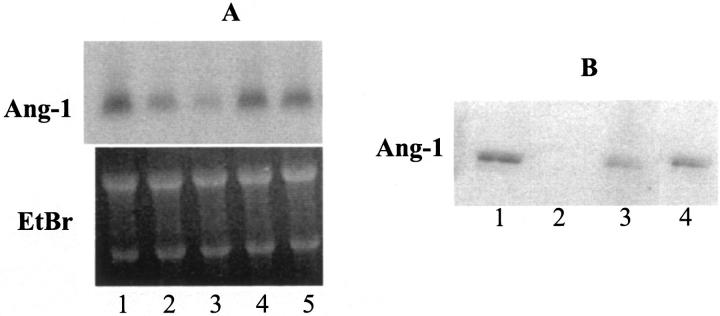
Because hypoxia is generally followed by reperfusion leading to free radical generation, we investigated whether oxidative stress may be involved in the generation of the fragile vessels and bleeding associated with LTPOC treatment. To test this hypothesis, HESCs were incubated under control or oxygen radical-generating conditions as described in Materials and Methods. Figure 6A ▶ indicates that the production of ROS decreased the expression of Ang-1 mRNA. The addition of the antioxidant desferrioxamine, a potent Fe-chelating agent, blocked the reduction in Ang-1 mRNA consistent with the agent’s ability to block ROS production 31 and inhibit oxidative damage. Similar results to those observed for mRNA were also observed for Ang-1 protein by Western blotting (Figure 6B) ▶ .
Figure 6.
Northern and Western blots for Ang-1 in control and ROS-treated HESCs. HESCs were treated under ROS-generating conditions for 24 hours as described in Materials and Methods. A: Northern blot of HESC-expressed Ang-1. Loading efficiencies were assessed by ethidium bromide staining of the 28S and 18S ribosomal RNA. Lane 1, control; lane 2, FeCl2 (50 μmol/L) + H2O2 (50 μmol/L); lane 3, FeCl2 (50 μmol/L) + EDTA (50 μmol/L) + H2O2 (50 μmol/L); lane 4, FeCl2 + H2O2 + desferrioxamine (250 μmol/L); and lane 5, FeCl2 + EDTA + H2O2 + desferrioxamine. Loading efficiencies were assessed by ethidium bromide staining of the 28S and 18S ribosomal RNA. B: Western blot of HESC-expressed Ang-1 protein. Lane 1, control; lane 2, FeCl2 (50 μmol/L) + H2O2 (50 μmol/L); lane 3, FeCl2 (50 μmol/L) + EDTA (50 μmol/L) + H2O2 (50 μmol/L); lane 4, FeCl2 + H2O2 + desferrioxamine (250 μmol/L).
In contrast, there was no discernable level of Ang-2 mRNA as assessed by either Northern blotting or by RT-PCR in either control or steroid-treated HESC cultures with or without hypoxia or oxidative stress (Figure 7) ▶ .
Figure 7.
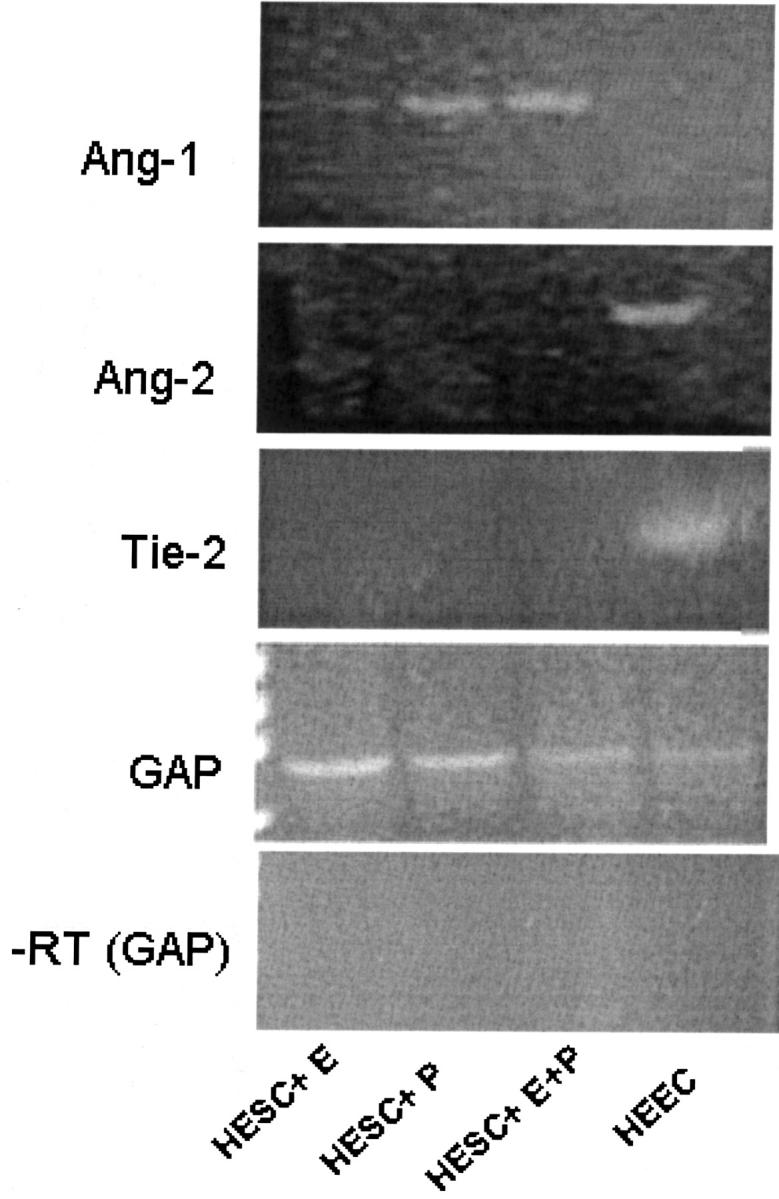
Ang-1, Ang-2, Tie-2, and GAPDH by RT-PCR from HEECs and HESCs RNA. Total RNA was extracted from untreated HEECs or HESCs treated with E2 (E), MPA (P), or E2 + MPA (E + P) and semiquantitative RT-PCR was conducted as described in Materials and Methods. The bottom panel shows the corresponding HESC and HEEC RNA in which reverse transcriptase was excluded from the reaction mixture. The primers used for this negative control reflect those of GAPDH (GAP). Similarly, no product was observed when reverse transcriptase was omitted and the reaction was performed with primers for Ang-1, Ang-2, or Tie-2 (not shown).
Effects of Hypoxia and Oxidative Stress on HEEC Ang-1 and Ang-2 Expression
Although a recent study reported the presence of steroid receptors in human endometrial endothelial cells (HEECs), 32 we and others have not been able to demonstrate these findings. 33,34 The lack of receptors was observed in either early passaged 33 or unpassaged endometrial endothelial cells (unpublished results). Hence, given their apparent lack of steroid receptors, HEECs were not treated with ovarian steroids. In contrast to the HESC cultures, HEECs failed to express detectable levels of Ang-1 mRNA after conducting RT-PCR. Conversely, they expressed Ang-2 mRNA whereas HESCs did not (Figure 7) ▶ . No signal was observed when the reaction was performed without the RT step. This is consistent with our previously published results demonstrating that HEECs expressed Ang-2 mRNA and these levels were greatly increased by hypoxia. 15
In addition, we demonstrate that as expected, HEECs but not HESCs display a band for the Tie-2 receptor. As a control, we show that GAPDH was expressed by all cells (Figure 7) ▶ .
Effects on the Activation of SAPK/JNK and p38 by Oxygen Radicals on Cultured Endometrial Cells
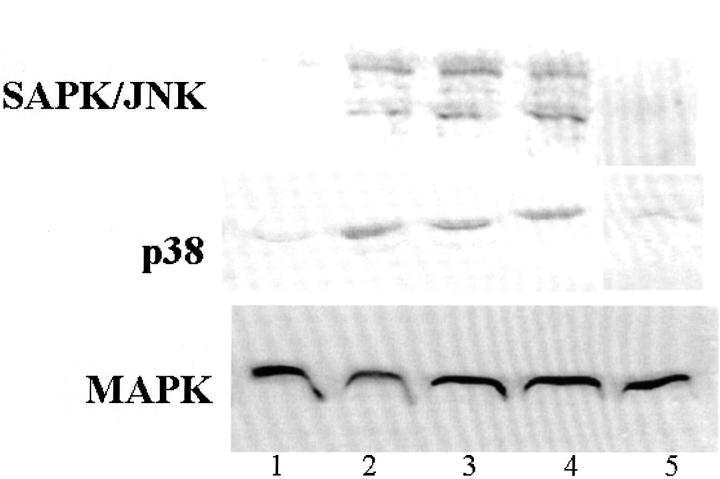
Cultured HESCs were incubated under control or oxygen radical-generating conditions. As can be seen in Figure 8 ▶ , Western blot analysis showed that SAPK/JNK and p38 were greatly activated (ie, phosphorylated) under these conditions whereas MAPK was not. The addition of the oxygen radical scavenger DMSO greatly decreased the activation of these kinases. Parallel blots were probed for total MAPK (unphosphorylated) demonstrating equal levels of protein in all samples.
Figure 8.
Western blots of SAPK/JNK and p38 after ROS treatment of HESCs. The activation (ie, phosphorylation) of p38 and SAPK/JNK in HESCs was detected after 30 minutes of ROS treatment as follows: lane 1, control; lane 2, H2O2 (50 μmol/L); lane 3, FeCl2 (50 μmol/L) + H2O2; lane 4, FeCl2 (50 μmol/L) + EDTA (50 μmol/L) + H2O2 (50 μmol/L); lane 5, FeCl2 + H2O2 + DMSO (28 mmol/L). Parallel blots were probed for total MAPK (unphosphorylated) demonstrating equal levels of protein in all samples and no effect by DMSO.
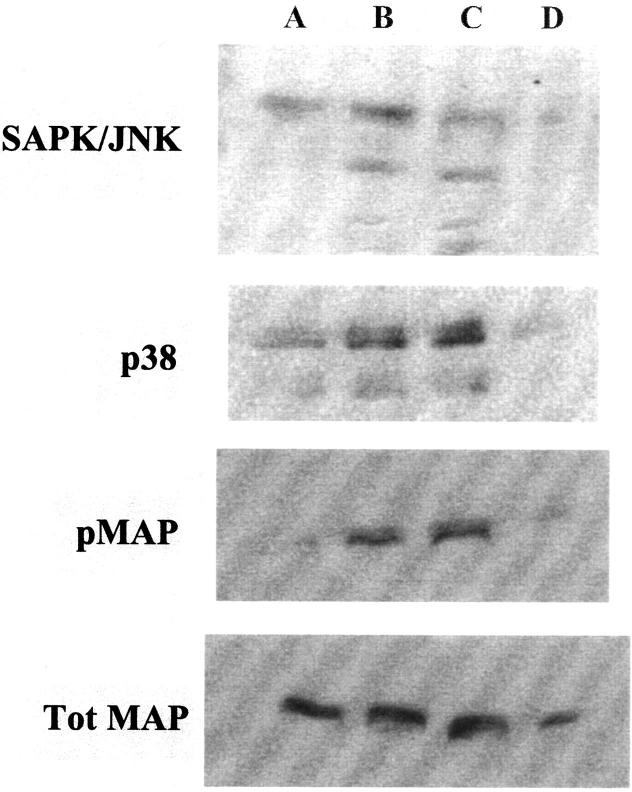
Similarly, Western blot analysis of cultured HEECs incubated under control or oxygen radical-generating conditions demonstrated that both SAPK/JNK and p38 were greatly activated by oxidative stress and DMSO decreased their activation (Figure 9) ▶ . In contrast to the effect on HESCs, oxidative stress resulted in the activation of MAPK in HEECs. The levels of total MAPK however, demonstrate similar loading levels.
Figure 9.
Western blots of SAPK/JNK, p38, and pMAP after ROS treatment of HEECs. The activation of p38, SAPK/JNK, and pMAP in HEECs was detected after 30 minutes of ROS treatment as follows: lane 1, control; lane 2, H2O2 (50 μmol/L); lane 3, FeCl2 (50 μmol/L) + H2O2; lane 4, FeCl2 + H2O2 + DMSO (28 mmol/L). Blots were stripped and reprobed for total MAPK.
Discussion
LTPOC treatment is often associated with vascular changes that result in increased numbers of abnormally enlarged fragile vessels and breakthrough bleeding. 2,6 However, the exact mechanism(s) regulating this aberrant angiogenesis are not clearly understood. The finding of a trend toward reduced perfusion in short-term progestrogen users suggests that LTPOC treatment results in hypoxia. 14 Tissue hypoxia is almost invariably followed by reperfusion-induced free radical damage. 18,35 It is now known that oxidative damage is involved in the pathogenesis of several human diseases. The classic example of organ damage related to ischemia reperfusion is represented by injury to the myocardium. 35,36 Indeed, reperfusion not only causes a rapid degeneration of endothelial cell function in the heart but also in the lung, gut, and kidney and has been linked to systemic sclerosis, Parkinson’s disease, and atherosclerosis. 37,38 Thus, ischemia/reperfusion are two critical events that may result from LTPOC treatment leading to the generation of oxidative stress, aberrant angiogenesis, and breakthrough bleeding. Importantly, it is now known that ROS play a major role in the activation of cell signaling pathways leading to vascular pathologies. 39,40
Indeed, SAPK/JNK activation has been shown in rat cardiac myocytes after hypoxia/reperfusion. 21,22 In cultured hepatocytes, hypoxia alone was able to induce JNK/SAPK activity, but not to the extent of that observed when hypoxia was followed by reperfusion. 23 Similarly, p38, another stress-activated kinase, has been shown to be activated in response to hypoxia in pulmonary microvascular endothelial cells 24 and by hypoxia/reperfusion in cultured cardiac myocytes. 22 Thus, the up-regulation of these pathways may represent a tool to identify free radical generation in affected tissues.
Our current findings demonstrated that proliferative and secretory endometrium display immunohistochemical staining for Ang-1. Although staining was observed in the vessels, RT-PCR conducted on total RNA isolated from cultured HEECs did not produce the Ang-1 fragment whereas the fragment was produced by HESCs. In contrast, HEECs expressed Ang-2 and Tie-2 mRNA (Figure 7) ▶ . Thus, it is possible that the staining pattern reflects binding of Ang-1 to its endothelial cell receptor, Tie-2. In contrast to the pattern of Ang-1 expression in cycling endometrium, an uneven pattern of immunostaining for Ang-1 was observed in endometrial tissue exposed to long-term LNG (local intrauterine or systemic delivery). It is important to note, that because of the difficulty in obtaining large numbers of tissues from LTPOC users, the immunohistochemical studies in the present report combined samples from patients treated with LNG delivered either as Norplant implants or as a LNG-releasing intrauterine system (LNG-IUS) and is limited to a total number of 16 specimens.
Consistent with the hypothesis that localized areas of hypoxia-reperfusion and the resultant formation of active oxygen species impair Ang-1 production after LTPOC, we show that both hypoxia and oxygen radical production decrease expression of Ang-1 mRNA and protein in cultured HESCs (Figures 5 and 6) ▶ ▶ . Thus, we posit that although progestin enhances cultured HESC Ang-1 expression in a normoxic, nonoxidative milieu, the inhibitory effects of LTPOC observed in vivo result from decreased endometrial perfusion, hypoxia/reperfusion, and paradoxically decreased expression of Ang-1. Furthermore, previous studies have shown hypoxia induced HEEC Ang-2 expression. 15 Increased Ang-2 promotes capillary branching and increased permeability, and decreased Ang-1 augments these effects and results in reduced vessel stabilization via the Tie-2 receptor that is expressed by the endothelial cells (Figure 7) ▶ . Thus, hypoxia/reperfusion and induction of free radicals could reduce the local endometrial Ang-1:Ang-2 ratio leading to aberrant angiogenesis and the observed abnormal vessels found in LTPOC-exposed endometria. This altered Ang-1:Ang-2 ratio would occur within the context of a simultaneous induction in VEGF expression, exacerbating the aberrant angiogenic stimulus and predisposition toward leaky vessels. 6,7
Further supporting our hypothesis that focal areas of hypoxia-reperfusion lead to the formation of active oxygen species after LTPOC treatment, is our finding of preferential activation of SAPK/JNK and p38, in LTPOC-exposed endometria (Figure 3) ▶ . In addition, the generation of oxygen radicals in vitro greatly activated SAPK/JNK and p38 in HESC and HEEC cultures (Figures 8 and 9) ▶ ▶ . Interestingly, MAPK was activated only in the endothelial but not the stromal cells after LTPOC treatment, consistent with results observed in cultured HEECs (Figure 9) ▶ .
Our present findings together with a previous study demonstrating increased circulating levels of lipid peroxides, concomitant depression of the antioxidant vitamin E, and a suggestion that vitamin E supplementation may counteract these unwanted side-effects in LTPOC users experiencing breakthrough bleeding 25 have clinical implications and suggest that antioxidant therapy may reduce the degree and extent of LTPOC-associated abnormal uterine bleeding.
Footnotes
Address reprint requests to Dr. Graciela Krikun, Department of Obstetrics and Gynecology, Yale University School of Medicine, 333 Cedars Street, New Haven, CT 06520-8063. E-mail: graciela.krikun@yale.edu.
Supported in part by a grant from the National Institutes of Health (RO1 HL33937-06 to C. J. L.).
Current address for G. Krikun, F. Schatz, R. Caze, and C. J. Lockwood is Department of Obstetrics and Gynecology, Yale University School of Medicine, 333 Cedars St., New Haven, CT 06520-8063.
References
- 1.Critchley HO, Wang H, Jones RL, Kelly RW, Drudy TA, Gebbie AE, Buckley CH, McNeilly AS, Glasier AF: Morphological and functional features of endometrial decidualization following long-term intrauterine levonorgestrel delivery. Hum Reprod 1998, 13:1218-1224 [DOI] [PubMed] [Google Scholar]
- 2.Hickey M, Simbar M, Young L, Markham R, Russell P, Fraser IS: A longitudinal study of changes in endometrial microvascular density in Norplant implant users. Contraception 1999, 59:123-129 [DOI] [PubMed] [Google Scholar]
- 3.Rogers PA, Au CL, Affandi B: Endometrial microvascular density during the normal menstrual cycle and following exposure to long-term levonorgestrel. Hum Reprod 1993, 8:1396-1404 [DOI] [PubMed] [Google Scholar]
- 4.Johannison E: Endometrial morphology during the normal cycle and under the influence of contraceptive steroids. dÁrcangues C Fraser IS Newton JR Odling V eds. Contraception and Mechanisms of Endometrial Bleeding. 1990:pp 53-81 Cambridge University Press, Cambridge
- 5.Runic R, Schatz F, Wan L, Demopoulos R, Krikun G, Lockwood CJ: Effects of norplant on endometrial tissue factor expression and vessel structure. J Clin Endocrinol Metab 2000, 85:3853-3859 [DOI] [PubMed] [Google Scholar]
- 6.Lebovic DI, Shifren JL, Ryan IP, Mueller MD, Korn AP, Darney PD, Taylor RN: Ovarian steroid and cytokine modulation of human endometrial angiogenesis. Hum Reprod 2000, 15(Suppl 3):67-77 [DOI] [PubMed] [Google Scholar]
- 7.Lau TM, Affandi B, Rogers PA: The effects of levonorgestrel implants on vascular endothelial growth factor expression in the endometrium. Mol Hum Reprod 1999, 5:57-63 [DOI] [PubMed] [Google Scholar]
- 8.Charnock-Jones DS, Macpherson AM, Archer DF, Leslie S, Makkink WK, Sharkey AM, Smith SK: The effect of progestins on vascular endothelial growth factor, oestrogen receptor and progesterone receptor immunoreactivity and endothelial cell density in human endometrium. Hum Rep 2000, 15(Suppl 3):85-95 [DOI] [PubMed] [Google Scholar]
- 9.Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD: Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell 1996, 87:1171-1180 [DOI] [PubMed] [Google Scholar]
- 10.Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J: Vascular-specific growth factors and blood vessel. Nature 2000, 407:242-248 [DOI] [PubMed] [Google Scholar]
- 11.Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD: Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 1997, 277:55-60 [DOI] [PubMed] [Google Scholar]
- 12.Oh H, Takagi H, Suzuma K, Otani A, Matsumura M, Honda Y: Hypoxia and vascular endothelial growth factor selectively up-regulate angiopoietin-2 in bovine microvascular endothelial cells. J Biol Chem 1999, 274:15732-15739 [DOI] [PubMed] [Google Scholar]
- 13.Mandriota SJ, Pyke C, Di Sanza C, Quinodoz P, Pittet B, Pepper MS: Hypoxia-inducible angiopoietin-2 expression is mimicked by iodonium compounds and occurs in the rat brain and skin in response to systemic hypoxia and tissue ischemia. Am J Pathol 2000, 156:2077-2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hickey M, Carati C, Manconi F, Gannon BJ, Dwarte D, Fraser IS: The measurement of endometrial perfusion in Norplant users: a pilot study. Hum Reprod 2000, 15:1086-1091 [DOI] [PubMed] [Google Scholar]
- 15.Krikun G, Schatz F, Finlay T, Kadner S, Mesia A, Gerrets R, Lockwood CJ: Expression of angiopoietin-2 by human endometrial endothelial cells: regulation by hypoxia and inflammation. Biochem Biophys Res Commun 2000, 275:159-163 [DOI] [PubMed] [Google Scholar]
- 16.Sharkey AM, Day K, McPherson A, Malik S, Licence D, Smith SK, Charnock-Jones DS: Vascular endothelial growth factor expression in human endometrium is regulated by hypoxia. J Clin Endocrinol Metab 2000, 85:402-409 [DOI] [PubMed] [Google Scholar]
- 17.Popovici RM, Irwin JC, Giaccia AJ, Giudice LC: Hypoxia and cAMP stimulate vascular endothelial growth factor (VEGF) in human endometrial stromal cells: potential relevance to menstruation and endometrial regeneration. J Clin Endocrinol Metab 1999, 84:2245-2248 [DOI] [PubMed] [Google Scholar]
- 18.Storey KB: Oxidative stress: animal adaptations in nature. Braz J Med Biol Res 1996, 29:1715-1733 [PubMed] [Google Scholar]
- 19.Bulger EM, Maier RV: Antioxidants in critical illness. Arch Surg 2001, 136:1201-1207 [DOI] [PubMed] [Google Scholar]
- 20.Ondiveeran HK, Fox-Robichaud A: New developments in the treatment of ischemia/reperfusion injury. Curr Opin Invest Drugs 2001, 2:783-791 [PubMed] [Google Scholar]
- 21.Kunz M, Ibrahim S, Koczan D, Thiesen HJ, Kohler HJ, Acker T, Plate KH, Ludwig S, Rapp UR, Brocker EB, van Muijen GN, Flory E, Gross G: Activation of c-Jun NH2-terminal kinase/stress-activated protein kinase (JNK/SAPK) is critical for hypoxia-induced apoptosis of human malignant melanoma. Cell Growth Differ 2001, 12:137-145 [PubMed] [Google Scholar]
- 22.Seko Y, Takahashi N, Tobe K, Kadowaki T, Yazaki Y: Hypoxia and hypoxia/reoxygenation activate p65PAK, p38 mitogen-activated protein kinase (MAPK), and stress-activated protein kinase (SAPK) in cultured rat cardiac myocytes. Biochem Biophys Res Commun 1997, 239:840-844 [DOI] [PubMed] [Google Scholar]
- 23.Crenesse D, Schmid-Alliana A, Hornoy J, Rossi B, Gugenheim J: Hypoxia-reoxygenation differentially stimulates stress-activated protein kinases in primary-cultured rat hepatocytes. Transplant Int 2000, 13:S597-S599 [DOI] [PubMed] [Google Scholar]
- 24.Kayyali US, Donaldson C, Huang H, Abdelnour R, Hassoun PM: Phosphorylation of xanthine dehydrogenase/oxidase in hypoxia. J Biol Chem 2001, 276:14359-14365 [DOI] [PubMed] [Google Scholar]
- 25.Subakir SB, Abdul Madjid O, Sabariah S, Affandi B: Oxidative stress, vitamin E and progestin breakthrough bleeding. Hum Reprod 2000, 15(Suppl 3):18-23 [DOI] [PubMed] [Google Scholar]
- 26.Noyes RW, Hertig AT, Rock J: Dating the endometrial biopsy. Fertil Steril 1950, 1:3-25 [DOI] [PubMed] [Google Scholar]
- 27.Krikun G, Schatz F, Mackman N, Guller S, Demopoulos R, Lockwood CJ: Regulation of tissue factor gene expression in human endometrium by transcription factors Sp1 and Sp3. Mol Endocrinol 2000, 14:393-400 [DOI] [PubMed] [Google Scholar]
- 28.Lockwood CJ, Krikun G, Runic R, Schwartz LB, Mesia AF, Schatz F: Progestin-epidermal growth factor regulation of tissue factor expression during decidualization of human endometrial stromal cells. J Clin Endocrinol Metab 2000, 85:297-301 [DOI] [PubMed] [Google Scholar]
- 29.Schatz F, Soderland C, Hendricks-Munoz KD, Gerrets RP, Lockwood CJ: Human endometrial endothelial cells: isolation, characterization, an inflammatory-mediated expression of tissue factor and type 1 plasminogen activator inhibitor. Biol Reprod 2000, 62:691-697 [DOI] [PubMed] [Google Scholar]
- 30.Kim I, Kim JH, Ryu YS, Liu M, Koh GY: Tumor necrosis factor-alpha upregulates angiopoietin-2 in human umbilical endothelial. Biochem Biophys Res Commun 2000, 269:361-365 [DOI] [PubMed] [Google Scholar]
- 31.Krikun G, Cederbaum AI: Stereochemical studies on the cytochrome P-450 and hydroxyl radical dependent pathways of 2-butanol oxidation by microsomes from chow-fed, phenobarbital-treated, and ethanol-treated rats. Biochemistry 1984, 23:5489-5494 [DOI] [PubMed] [Google Scholar]
- 32.Iruela-Arispe ML, Rodriguez-Manzaneque JC, Abu-Jawdeh G: Endometrial endothelial cells express estrogen and progesterone receptors and exhibit a tissue specific response to angiogenic growth factors. Microcirculation 1999, 6:127-140 [PubMed] [Google Scholar]
- 33.Schachter B, Schatz F, Soderland C, Krey L: Do endothelial cells of human endometrial microvessels express estrogen receptors? Soc Gynecol Invest 1997, 4:125A [Google Scholar]
- 34.Kohnen G, Campbell S, Jeffers MD, Cameron IT: Spatially regulated differentiation of endometrial vascular smooth muscle cells. Hum Reprod 2000, 15:284-292 [DOI] [PubMed] [Google Scholar]
- 35.Vendemiale G, Grattagliano I, Altomare E: An update on the role of free radicals and antioxidant defense in human disease. Int J Clin Lab Res 1999, 29:49-55 [DOI] [PubMed] [Google Scholar]
- 36.Hammond B, Kontos HA, Hess ML: Oxygen radicals in the adult respiratory distress syndrome, in myocardial ischemia and reperfusion injury, and in cerebral vascular damage. Can J Physiol Pharmacol 1985, 63:173-187 [DOI] [PubMed] [Google Scholar]
- 37.Simonini G, Pignone A, Generini S, Falcini F, Matucci C: Emerging potentials for an antioxidant therapy as a new approach to the treatment of systemic sclerosis. Toxicology 2000, 155:1-15 [DOI] [PubMed] [Google Scholar]
- 38.Berg D, Gerlach M, Youdim MB, Double KL, Zecca L, Riederer P, Becker G: Brain iron pathways and their relevance to Parkinson’s disease. J Neurochem 2001, 79:225-236 [DOI] [PubMed] [Google Scholar]
- 39.Kehrer JP: The Haber-Weiss reaction and mechanisms of toxicity. Toxicology 2000, 149:43-50 [DOI] [PubMed] [Google Scholar]
- 40.Lounsbury KM, Hu Q, Ziegelstein RC: Calcium signaling and oxidant stress in the vasculature. Free Radic Biol Med 2000, 28:1362-1369 [DOI] [PubMed] [Google Scholar]