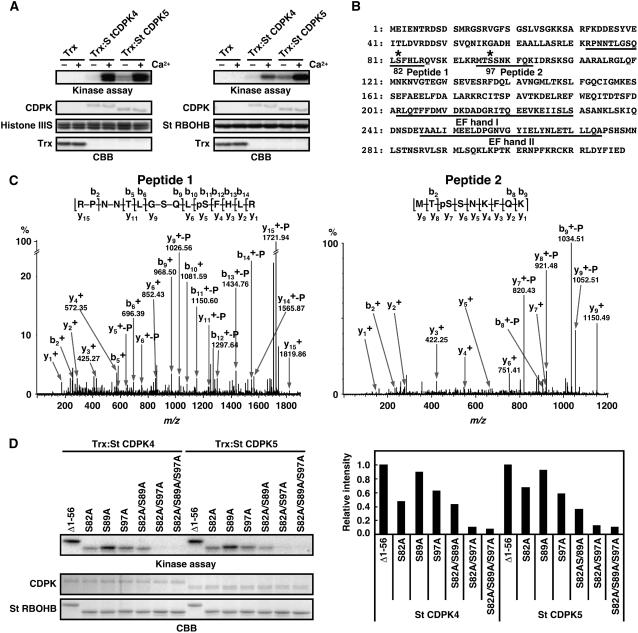
Figure 4.
Phosphorylation of Ser-82 and Ser-97 of St RBOHB by Recombinant St CDPK4 and St CDPK5 Proteins.
(A) Purified N-terminal peptides of St RBOHB1-318 (right) or histone III-S (left) were used as substrates for Trx-fused St CDPK4 and St CDPK5. Phosphorylation of St RBOHB and histone III-S was detected by x-ray film. Protein loads were monitored by CBB staining.
(B) Amino acid sequence of St RBOHB. Fragments confirmed as phosphopeptide and EF-hand motifs are underlined. Asterisks indicate phosphorylation sites.
(C) Hybrid quadrupole/time-of-flight tandem mass spectrometry spectra identifying phosphorylation sites of N-terminal peptides of St RBOHB1-318 in vitro. The phosphorylated proteins were separated by SDS-PAGE and in-gel digested with trypsin. Resulting peptides were extracted and analyzed by LC-MS/MS analysis on a capillary LC system coupled directly to a Waters Q-ToF Ultima mass spectrometer. Identified phosphoseryl residues are denoted as pS. The superscript + indicates singly protonated ions. −P indicates neutral loss of H3PO4 from phosphorylated peptides.
(D) Effects of mutations of Ser-82 and Ser-97 residues on phosphorylation in vitro. N-terminal peptides of St RBOHB (Δ1-56)–introduced amino acid substitution in Ser-82, Ser-89, and/or Ser-97 were used as substrates for Trx-fused St CDPK4 and St CDPK5. The difference in size between Δ1-56 and Δ1-56 carrying point mutations is derived from different lengths of additional vector sequences by use of distinct restriction sites. Phosphorylation was detected by x-ray film (top panel). Protein loads were determined by CBB staining (middle panel). Incorporation of radioactivities into the various N-terminal regions of St RBOHB was measured using a phosphor imager (bottom panel).