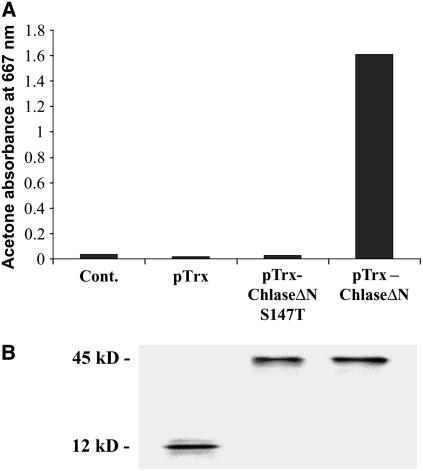
Figure 2.
In Vitro Enzyme Activity of Recombinant Chlase Versions.
Citrus Chlase versions were expressed as recombinant proteins in E. coli. Total soluble proteins were extracted from E. coli harboring pTrxFus plasmid expressing thioredoxin (pTrx; 12 kD), pTrxFus-Chlase plasmid containing the mature form of Chlase (lacking the N-terminal 21 amino acids) fused to thioredoxin (pTrx-ChlaseΔN; 45 kD), or pTrxFus-Chlase-S147T plasmid containing a version of ChlaseΔN bearing a Ser-to-Thr point mutation at position 147 (pTrx-ChlaseΔN-S147T; 45 kD). Protein extracts, and a protein-free reaction control, were examined for Chlase in vitro activity (A) as described in Methods and for Chlase-thioredoxin recombinant fusion protein level by protein gel blot analysis (B) using thioredoxin-specific antibodies.