Figure 1.
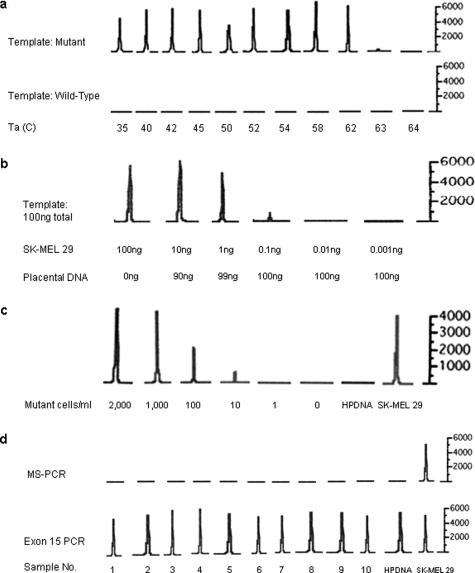
Sensitivity and specificity of the MS-PCR assay. a: Annealing temperature gradient. Annealing temperatures between 35 and 64°C were tested. The amplified products were analyzed using an ABI 310 Genetic Analyzer, and the resulting electropherograms are presented. In the top row, amplification of templates containing the BRAF mutation is markedly reduced or absent at annealing temperatures of 63°C or higher. At all temperatures, the reaction failed to amplify the wild-type control (bottom row). The mutant template consisted of DNA purified from the BRAF mutant melanoma cell line SK-MEL 29; the wild-type template consisted of human placental DNA. The y axis is relative fluorescence units. b: Mixing experiment. The specified quantities of DNA from the BRAF mutant cell line SK-MEL 29 were mixed with human placental DNA (wild-type BRAF) to a sum of 100 ng of DNA. The mixture was used as a template for the MS-PCR. The limit of detection for mutant BRAF alleles using this assay was 0.1 ng of cell line DNA. c: Dilution experiment. Normal blood samples (5 ml each) were spiked with decreasing numbers of SK-MEL 29 cells, mutant for BRAF. Peripheral blood mononuclear cells were isolated, and DNA was extracted. The lowest concentration for which a peak is present is 10 cells/ml. Mutant cells were undetectable at concentrations below this and in the negative control human placental DNA (HPDNA). d: Negative controls. Ten peripheral blood samples from normal volunteers were analyzed by MS-PCR. None of the samples had detectable mutant BRAF alleles (top panel). In the bottom panel, the entire BRAF exon 15 was amplified (as expected) from all volunteer samples and from human placental (HPDNA) and SK-MEL 29 control DNA samples.