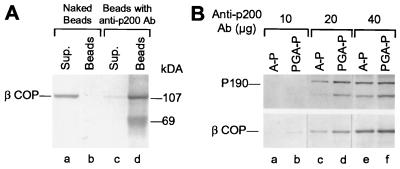
Figure 4.
Anti-P200 mAb bound to PGA beads removes native coatomer from an LCP fraction. (A) LCP aliquots (200 μg) were incubated for 40 hr at 4°C with either naked PGA beads (lanes a and b), or with beads containing anti-P200 antibody (lanes c and d). The beads (lanes b and d), recovered by centrifugation and washed in immunoprecipitation buffer (20 mM Hepes⋅KOH, pH 7.2/100 mM KCl/1 mM DTT), and the supernatants combined with the washes (lanes a and c), were analyzed by SDS/PAGE and Western blotting with the anti-βCOP M3A5 mAb. (B) Immunoadsorption of coatomer by affinity purified anti-P200 antibody. Beads containing the indicated amounts of anti-P200 antibody, purified by binding to PGA (PGA-P, lanes b, d, and f), or by affinity purification on immobilized P200 (A-P, lanes a, c, and e), were incubated with 1 mg LCP for 20 hr at 4°C. After three washes with cold immunoprecipitation buffer, the bound proteins were analyzed by SDS/PAGE and Western blotting with the anti-P200 (Top) or the anti-βCOP M3A5 antibody (Bottom). The band below P190 detected by the AD7 antibody corresponds to a degradation product of P190.