Abstract
The ribosomal S6 protein kinase p70 S6 kinase is known for its role in modulating cell-cycle progression, cell size, and cell survival. In response to mitogen stimulation, p70 S6 kinase activation up-regulates ribosomal biosynthesis and enhances the translational capacity of the cell. In Alzheimer’s disease (AD), there is a marked increase in total tau protein in the form of abnormally hyperphosphorylated tau (PHF-tau) in neurons with neurofibrillary tangles (NFTs). In the present study, we investigated whether p70 S6 kinase activation is associated with PHF-tau accumulation in AD. By immunohistochemistry, we found that the levels of phosphorylated p70 S6 kinase (at Thr389 or at Thr421/Ser424) were increased in accordance with the progressive sequence of neurofibrillary changes according to Braak’s criteria. Confocal microscopy showed that in AD brain, phosphorylated p70 S6 kinase appeared especially in neurons that are known to later develop NFTs. This pattern of neurons showed dot-like structures of phosphorylated p70 S6 kinase and hyperphosphorylated tau, which partially correlated with rab5 (endosome marker), lamp-1 (lysosome marker), and ubiquitin (ubiquitin-proteasomal system marker). By indirect enzyme-linked immunosorbent assay, phosphorylated p70 S6 kinase (Thr389 or Thr421/Ser424), total tau, and PHF-tau were found to be significantly increased in AD brain as compared to control cases. The levels of total p70 S6 kinase and p70 S6 kinase phosphorylated at Thr421/Ser424 showed significant correlations with the levels of both total tau and PHF-tau. Regression analyses revealed a significant dependence of total tau or PHF-tau on p70 S6 kinase phosphorylated at Thr421/Ser424 rather than at Thr389. The levels of ribosomal protein S6 as well as the levels of markers for the proteolytic system were also significantly increased in AD as compared to control brain. Using a SH-SY5Y neuroblastoma cell model, we found that 100 μmol/L zinc sulfate could induce p70 S6 kinase phosphorylation and activation, in particular at Thr421/Ser424. This up-regulation of the activated kinase resulted in an increased expression and phosphorylation of tau. Pretreatment of cells with rapamycin (an inhibitor of FRAP/mTOR which is the immediate upstream kinase of the p70 S6 kinase) attenuated the effects induced by zinc. In primary cultured neurons of rat cortical cortex, zinc sulfate treatment could repeat p70 S6 kinase phosphorylation and activation at Thr421/Ser424, followed by increased expression and phosphorylation of tau. Taken together, these data suggest that activated p70 S6 kinase could mediate an up-regulation of tau translation. The partial co-localization of phosphorylated p70 S6 kinase with rab5, lamp-1 and ubiquitin, or PHF-tau with ubiquitin suggests that the activated proteolytic system might not be sufficient to degrade the over-produced and over-phosphorylated tau protein. A p70 S6 kinase modulated up-regulation of tau translation might contribute to PHF-tau accumulation in neurons with neurofibrillary changes.
Alzheimer’s disease (AD) is a complex neurodegenerative disorder characterized by a progressive and hierarchic decline in cognitive function. One of the major lesions in AD brain is the formation of paired helical filaments (PHFs) that are mainly composed of abnormally hyperphosphorylated microtubule-associated protein tau (PHF-tau). 1-5 This neurofibrillary pathology is seen as neurofibrillary tangles (NFTs), neuropil threads, and dystrophic neurites surrounding the extracellular deposits of β-amyloid in neuritic plaques. In AD brain, there is a marked increase in total tau and the increase is in the form of PHF-tau. 6,7 A significant amount (∼60%) of normal tau remains in the 100,000 × g supernatant of AD brain, as compared to that of controls. 6,7 The increase of total tau in AD brain might indicate that to keep the cell functioning, neurons enduring neurofibrillary degeneration still efficiently produce tau protein to compensate for that being converted to PHF-tau. Such PHF-tau fails to promote assembly and stabilize microtubules. 8,9 An up-regulation of translational capacity and or a decreased turnover might in theory contribute to an increased level of total tau and the formation of PHF-tau in tangle-bearing neurons.
One efficient up-regulator of the cell translational capacity is p70 S6 kinase. P70 S6 kinase is one of two isoforms of ribosomal S6 kinase 1, the other isoform being p85 S6 kinase. P70/85 S6 kinases are formed from the same transcript by two different translation start sites. 10,11 P70 S6 kinase is largely cytoplasmic. 11,12 In contrast, p85 S6 kinase appears to be exclusively nuclear owing to an additional 23-amino acid sequence in the amino terminus, which functions as a nuclear localization signal. 11,12 Activation of ribosomal S6 kinase 1 depends on its level of phosphorylation state at eight sites: Thr (T) 229, Ser (S) 371, T389, S404, S411, S418, T421, and S424. 13 Of these, S411, S418, T421, and S424 are located in the autoinhibitory domain and S371, T389, and S404 lie in the linker domain. The T229 that lies in the catalytic domain plays a key role in modulating ribosomal S6 kinase 1 activity. Ribosomal S6 kinase 1 activation may be achieved by sequential phosphorylation of these sites by mitogen-activated protein kinase (MAPK) pathways and phosphoinositol 3 kinase (PI3K). 14-17 Ribosomal S6 kinase 1 activation up-regulates ribosomal biosynthesis, and enhances cell translational capacity through phosphorylation of the 40 S ribosomal protein S6 (rpS6) and regulation of mRNAs with 5′-terminal oligopyrimidine tracts (5′TOP). 18,19 As such, ribosomal S6 kinase 1 has been implicated in regulation of cell-cycle control, cell size, cell differentiation, and cell motility. 20,21
To address whether PHF-tau accumulation might involve p70 S6 kinase-mediated translation of tau, the immunoreactivity of phosphorylated p70 S6 kinase was investigated in brains at different Braak’s stages of the disease, showing different frequencies and topographical distributions of neurofibrillary changes. 22 The stages defined by this system span the pre-clinical and symptomatic phases, which correspond to the progressive accumulation and wide dissemination of NFTs through the brain at the end stages of disease. Using antibodies to phosphorylated (active) p70 S6 kinase, we examined the distribution of the active form of p70 S6 kinase in brains with different extents of neurofibrillary changes. 22 We found that activated p70 S6 kinase is co-distributed with neurofibrillary pathology in a predictable sequence from the entorhinal cortex, to the hippocampal CA1 and layers III and V of the temporal cortex. Activated p70 S6 kinase was obviously increased in neurons before developing NFTs. Only levels of activated p70 S6 kinase phosphorylated at T421/S424 showed a dependent correlation with total tau and PHF-tau levels.
Recently, it has been shown that zinc ions and hydrogen peroxide are able to activate p70 S6 kinase through PI3K and MAPK signaling pathways. 23-25 Zinc ions could also stimulate the activity of the protein kinase mammalian target of rapamycin (mTOR, also known as FRAP and RAFT), an important upstream kinase of p70 S6 kinase. 26 Several studies have shown that zinc levels are elevated in brain regions such as the hippocampus and amygdala that are heavily affected by AD pathology. 27-29 To clarify the relationship between p70 S6 kinase and tau, p70 S6 kinase activity was modulated by zinc sulfate, and the effects on tau synthesis and phosphorylation were investigated in SH-SY5Y neuroblastoma cells. A 30-minute treatment with 100 μmol/L zinc sulfate resulted in increased p70 S6 kinase phosphorylation and activation, in particular at T421/S424 sites. This was followed by increased levels of total, normal, and hyperphosphorylated tau. Pretreatment of cells with rapamycin before zinc sulfate produced a threefold decrease of p70 S6 kinase phosphorylated at T421/424. This was followed by significant reductions of total, normal, and hyperphosphorylated tau levels. Similar results were repeatedly found in primary cultured neurons.
Our data indicate that aberrant activation of p70 S6 kinase might be involved in up-regulated translation and deposition of tau protein as PHF-tau in AD.
Materials and Methods
Materials
Cytosolic abnormally hyperphosphorylated tau (AD p-tau) and PHF-tau were isolated from an AD brain as described previously. 30 Extracts from non-treated and serum-treated NIH-3T3 cells, affinity-purified polyclonal rabbit antibodies against p70 S6 kinase phosphorylated at T389 or at T421/S424, and to control p70 S6 kinase were purchased from Cell Signaling Technology (Beverly, MA). Rabbit antiserum 134 days to longest isoform of recombinant human tau was raised in our laboratory. 31 Mouse monoclonal (mAb) AT8 was purchased from Innogenetics (anti-human PHF-tau; Zwujndrecht, Belgium). mAb PHF-1 was a gift from Dr. Peter Davies, Albert Einstein College of Medicine (Bronx, NY) and mAb Tau-1 from Dr. L. Binder, North Western University (Chicago, Illinois). Rab5 (an early endosome marker) and lamp-1 (a lysosome marker) were bought from Transduction Laboratories (Lexington, KY). mAb anti-KDEL was bought from Stressgen Biotechnology (Victoria, B.C., Canada). Rabbit polyclonal antibody to ubiquitin and mAb NCL-UBIQm were bought from Novocastra Laboratories (Newcastle upon Tyne, UK). For detailed information on antibodies used, see Table 1 ▶ . Zinc sulfate, rapamycin and protease-inhibitor cocktail for mammalian cell and tissue extracts were bought from σ-Aldrich (St. Louis, MO).
Table 1.
Primary Antibodies Used in the Study
| Antibody | Specificity | Phosphorylation sites | Dilution | Reference no. or sources |
|---|---|---|---|---|
| p70 S6K | Total p70 S6K | 1:100–1:500 | Cell signaling | |
| p70 S6K (T389) | P, active p70/p85 S6K | T389 | 1:100–1:500 | Cell signaling |
| p70 S6K (T421/S424) | P, active p70/p85 S6K | T421/S424 | 1:100–1:500 | Cell signaling |
| S6 (total) | P and NP S6 | 1:250 | Cell signaling | |
| S6 (S235/236) | P, active S6 | S235/236 | 1:250 | Cell signaling |
| S6 (S240/244) | P, active S6 | S240/244 | 1:250 | Cell signaling |
| R134d | P tau and NP tau | Phospho-independent | 1:2500 | 31 |
| Tau-1 | NP tau | S198/199/202/T205 | 1:20,000–40,000 | 79 |
| PHF-1 | P tau | S396/404 | 1:200–1:400 | 80 |
| AT8 | P tau | S202/T205 | 1:500–1:2000 | 81 |
| Rab5 | Early endosomes | 1:400 | Transduction Laboratories | |
| Lamp-1 | Lysosomes | 1:200 | Transduction Laboratories | |
| MAb anti-KDEL | Endoplasmic reticulum | 1:20 | Stressgen Biotechnology | |
| MAb NCL-UBIQm | Ubiquitin | 1:10 | Novocastra Laboratories | |
| Rabbit polyclonal antibody ubiquitin | Ubiquitin | 1:20 | Novocastra Laboratories |
P, phosphorylated epitope; NP, nonphosphorylated epitope.
Brain Staging
Tissue blocks from 16 individuals, ages 50 to 93 years, were obtained at autopsy (for case details, see Table 2 ▶ ) from the temporal lobe, including the entorhinal, hippocampal, and temporal cortices and/or amygdala, and fixed by immersion in a mixture of 4% paraformaldehyde and picric acid at pH 7.0. 32 The frozen tissue blocks and those embedded in polyethylene glycol (PEG) were sectioned at 50 to 100 μm. Aldehyde fuchsin-D arrow red staining was used for topographic orientation. 33 Neurofibrillary changes were demonstrated by the Gallyas silver-iodide technique 34 and by immunohistochemistry with mAb AT8. 35 Amyloid deposits were visualized using selective silver staining. 36
Table 2.
Detailed Information of Cases Used for Immunohistochemistry
| Case | Sex | Age | Braak’s staging | |
|---|---|---|---|---|
| NFT | β-Amyloid | |||
| 1 | F | 50 | 0 | 0 |
| 2 | M | 55 | 0 | 0 |
| 3 | M | 62 | 0 | 0 |
| 4 | M | 67 | 0 | 0 |
| 5 | M | 53 | I | 0 |
| 6 | M | 70 | I–II | 0 |
| 7 | F | 68 | I–II | 0 |
| 8 | F | 66 | II | 0 |
| 9 | F | 86 | III | 0 |
| 10 | F | 86 | III | 0 |
| 11 | F | 93 | IV | 0 |
| 12 | F | 85 | IV | A |
| 13 | N/A | N/A | V | C |
| 14 | F | 60 | V | C |
| 15 | M | 75 | V | C |
| 16 | N/A | 91 | V | C |
N/A, Not available.
All cases were classified according to the histopathological staging system for neurofibrillary changes and amyloid deposition described previously. 22,37 This staging procedure permits the differentiation of controls from six stages with increasing severity of neurofibrillary changes located mainly in the entorhinal cortex/hippocampal formation (Table 2) ▶ . The transentorhinal stage I is defined by the selective presence of NFTs and numerous dendritic neuropil threads in projecting cells within the transentorhinal region. Accentuated transentorhinal pathology as well as very mild involvement of the entorhinal layer Pre-α and Ammon’s horn sector CA1 are seen in the transentorhinal stage II. The limbic stages III/IV show, in addition to lesions in the entorhinal region, severe abnormalities in the entorhinal region and hippocampal formation, with expansion of changes within the limbic system. The isocortical stages V/VI are characterized by severe, widespread destruction in limbic regions, as well as involvement of isocortical association areas.
The 16 cases were also classified with respect to the extent of amyloid deposition. 22 In accordance with this classification, the term “amyloid” refers to plaque-like deposits with or without a neuritic component. Stage 0 is characterized by the absence of amyloid deposits. Stage A cases show a few plaques in the basal isocortex. Stage B cases show many plaques in the basal isocortex and allocortex, whereas stage C cases display large numbers of plaques in all parts of the cortex.
Immunohistochemistry
Tissue sections were incubated for 40 to 44 hours at 4°C with mAb AT8 at a dilution of 1:2000 and rabbit antibodies to p70 S6 kinase phosphorylated at T389 or T421/S424 at 1:100. This was followed by incubation with biotinylated anti-mouse IgM or anti-rabbit IgG at a dilution of 1:200 for 2 hours and visualization with the avidin-biotin-peroxidase complex kit (Vector, Burlingame, CA) with 3–3′-diaminobenzidine-4 HCl/H2O2 (DAB; Sigma, St. Louis, MO) as a substrate. The images of p70 S6 kinase phosphorylated at T389 or T421/S424 were taken by confocal microscope using the transmission function of the Lasersharp software in BioRad Laser Scanning Confocal Imaging System (Radiance Plus; Bio-Rod House, Hertfordshire, UK), and composed using Adobe Photoshop 5.0 software.
Double-Immunofluorescent Staining and Confocal Microscopy
Double-immunofluorescent staining of floating sections was carried out using CY3-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) to stain bound antibodies to p70 S6 kinase phosphorylated at T389 or T421/S424, and CY2-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories, Inc.) to stain bound mAb AT8. For double-staining of polyclonal antibody to p70 S6 kinase at T421/S424 and mAbs Rab5, lamp-1, and ubiquitin, or of polyclonal antibody to ubiquitin and AT8/PHF-tau, 30-μm formalin-fixed frozen sections were used. CY2 and CY5-conjugated secondary antibodies were used to stain bound mAbs or polyclonal antibodies, respectively. A BioRad Laser Scanning Confocal Imaging System (Radiance Plus) was used to determine co-localization of the CY3 (red)- or CY5 (far red)-labeled p70 S6 kinase at T389 or T421/T424 to CY2 (green)-labeled monoclonal antibodies. The system is equipped with a Nikon Eclipse inverted microscope (TE300). An argon ion laser that excites at 488 nm with a dichroic beamsplitter 560DCLP and a bandpass filter HQ515/30 was used to detect CY2 (green)-labeled monoclonal antibodies. A HeNe laser that excites at 543 nm with E570LP emission filter was used to measure p70 S6 kinase at T389 or T421/S424 labeled by CY3 (red). To detect the CY5 (far red) signal, a Diode laser that excites at 638 nm with E660LP emission filter was used. Laser light illuminates a Nikon 60×/1.4 NA oil immersion objective. Images scanned on the two channels (red and green) were merged to produce a single profile. Fluorescence images were collected at 1× or 2.5× zoom using the BioRad Lasersharp Software Package, and processed using Adobe Photoshop 5.0.
Homogenate Preparation and Protein Measurement
For homogenate preparation, tissue blocks of the medial temporal cortex from 13 control and 22 AD brains were used. Gray matter was separated from white matter and homogenized in 10 volumes (ml/g wet tissue) of protease-inhibitor cocktail buffer (Sigma) over ice. Protein concentrations of all samples were quantitated by the method of Bradford 38 and samples were then stored at −70°C.
Western Blotting
After boiling homogenates for 4 minutes in loading buffer containing Tris-HCl 62.5 mmol/L, 20% glycerol, 2% sodium dodecyl sulfate, 4% β-mercaptoethanol, and 0.02% bromophenol, pH 7.4, the samples were loaded and proteins resolved by 10% SDS-PAGE for 45 minutes. Proteins were then transferred to nitrocellulose transfer membranes and probed with primary antibodies to p70 S6 kinase and tau. After blocking with 5% (w/v) nonfat milk solution, the primary antibodies were detected using horseradish peroxidase-linked anti-rabbit or anti-mouse IgGs (Amersham Biosciences AB, Uppsala, Sweden) at 1:2000, and detected by the enhanced chemiluminescence kit (Amersham Biosciences AB).
Indirect Enzyme-Linked Immunosorbent Assay (ELISA)
The levels of control p70 S6 kinase, p70 S6 kinase phosphorylated at T389 or T421/S424, and tau, as well as rpS6 (total, phosphorylated at S235/236 and S240/244) were measured by indirect ELISA in homogenates of 13 control and 22 AD cases (Table 3) ▶ . Microtiter plates (Nunc-Immuno, Roskilde, Denmark) were coated in duplicates with 2 μg of protein/well in 20 mmol/L Tris-HCl, pH 7.4, buffered with 2.5 mmol/L ethylenediaminetetraacetate (EDTA), 2.5 mmol/L EGTA, 0.1% (v/v) protease-inhibitor cocktail (Sigma-Aldrich, Stockholm, Sweden), 0.1% sodium azide, overnight at 4°C. Unspecific protein-binding sites were blocked with 3% BSA in 10 mmol/L Tris-HCl buffer with 0.85% NaCl and 0.2% Tween-20 for 1 hour at room temperature. The plates were incubated with rabbit antibodies to total p70 S6 kinase and p70 S6 kinase phosphorylated at T389 or T421/T424 (1:200), to total tau (R134, 1:2500), and to rpS6 (total) and S6 phosphorylated at S235/236 or S240/244 (1:250), or with mAbs to tau unphosphorylated at S198/199/202/T205 (Tau-1, 1:40,000), tau phosphorylated at S202/T205 (AT8, 1:500) and at S396/404 (PHF-1, 1:200) overnight at 4°C. Bound rabbit or mouse antibodies were detected with peroxidase-conjugated anti-rabbit or anti-mouse IgG (1:100 to 1:2000; Jackson ImmunoResearch Laboratories, Inc.), followed by reaction for 10 to 30 minutes with tetramethylbenzidine (TMB) substrate solution (SMS-gruppen, Denmark). After stopping reaction with 100 μl/well 1 mol/L phosphoric acid, absorbances were measured at 450 nm using a Spectra Max 250 ELISA reader (Molecular Devices, Sunnyvale, CA).
Table 3.
Detailed Information of Cases Used for Western Blotting and ELISA Analysis
| Case | Sex | Age | Diagnosis | Postmortem delay (hours) |
|---|---|---|---|---|
| 1 | M | 67 | Control | 2 |
| 2 | M | 75 | Control | 10 |
| 3 | F | 84 | Control | 16 |
| 4 | M | 79 | Control | 17 |
| 5 | M | 82 | Control | 22 |
| 6 | M | 76 | Control | 3 |
| 7 | M | 84 | Control | 7 |
| 8 | M | 83 | Control | 8 |
| 9 | F | 98 | Control | 9 |
| 10 | F | 96 | Control | 10 |
| 11 | M | 79 | Control | 5 |
| 12 | F | 84 | Control | 7 |
| 13 | F | 82 | Control | 4 |
| 14 | F | 90 | AD | 10 |
| 15 | M | 86 | AD | 7 |
| 16 | M | 71 | AD | 7 |
| 17 | M | 88 | AD | 7 |
| 18 | F | 73 | AD | 7 |
| 19 | M | 82 | AD | 6 |
| 20 | F | 100 | AD | 6 |
| 21 | F | 92 | AD | 6 |
| 22 | F | 78 | AD | 6 |
| 23 | F | 97 | AD | 9 |
| 24 | F | 91 | AD | 4 |
| 25 | F | 84 | AD | 4 |
| 26 | F | 87 | AD | 5 |
| 27 | F | 54 | AD | 3 |
| 28 | F | 76 | AD | 6 |
| 29 | F | 82 | AD | 7 |
| 30 | F | 74 | AD | 6 |
| 31 | F | 84 | AD | 7 |
| 32 | F | 84 | AD | 2 |
| 33 | F | 68 | AD | 4 |
| 34 | F | 74 | AD | 4 |
| 35 | F | 86 | AD | 3 |
Average age (years): Control, 82.23 ± 8.25; AD, 81.86 ± 10.38 (P >0.05 vs. control). Average postmortem delay (hours): Control, 9.23 ± 5.9; AD, 5.73 ± 1.96 (P >0.05 vs. control).
Cell Culture, Treatment, and Cell Extract Preparation
SH-SY5Y human neuroblastoma cells were grown to 70 to 80% confluence in 100-mm-diameter dishes or 6-well culture plates in an atmosphere of 5% CO2/95% air at 37°C, using Dulbecco’s modified Eagle’s medium (DMEM)/F12 medium (1:1) supplemented with 5% fetal bovine serum (FBS), 100 units/ml penicillin, and 0.1 mg/ml streptomycin. The cells were then cultured in 0.5% FBS media for two days. Before treating the cells with zinc sulfate, the cultures were kept in fresh serum-free medium for two hours to reduce the basal level of p70 S6 kinase phosphorylation. Cells were treated with zinc sulfate with or without 1 hour of rapamycin pretreatment (20 ng/ml). After being washed with ice-cold phosphate-buffered saline, the cells were harvested and suspended in cell lysate buffer containing 2 mmol/L EGTA, 25 mmol/L NaF, 200 μmol/L NaVO4, 0.5 mmol/L phenylmethylsulfonyl fluoride, 5 mmol/L EDTA, 150 mmol/L NaCl, 50 mmol/L Tris-HCl (pH 7.4), Triton X-100, protease-inhibitor cocktail (1:200) and kept on ice for 20 minutes. The cell lysates were kept at −80°C. These samples were used for Western blotting. The immunoblots were stripped as described by the nitrocellulose membrane manufacturer (Amersham Biosciences AB) and re-blotted with other antibodies. The relative density of immunoreactive bands on Western blots was calculated from the optical density multiplied by the area of the selected band, following acquisition of the blot image through Image Master (Amersham Biosciences AB).
For primary cultures, tissues from Sprague-Dawley rat brains at embryonic 16- to 18-days-old were used. Briefly, cells were taken from the cortical cortice of pups, seeded in 6-well plates or 100-mm dishes and cultured in Neurobasal media supplemented with 1% B-27 (Invitrogen, Carlsbad, CA). Half of the culture media was changed every 3 days. Fresh media were supplied to the cells and incubated for 12 hours before zinc sulfate treatment (200 μmol/L) with or without rapamycin (20 ng/ml) pretreatment. The samples were collected and Western blotting was performed as above. Primary cultured neurons were treated at 6 to 8 days in vitro.
Statistical Analysis
Statistical analysis was performed using SPSS 9.0 (SPSS Inc., Chicago, IL). Levels of p70 S6 kinase, tau proteins, proteolytic system markers, and rpS6 were compared in brain homogenates between AD and control groups with Student’s independent t-test. The Pearson correlation and stepwise regression between p70 S6 kinase and tau levels were also analyzed. For regression analysis, the variable entered/removed criteria were probability-of-F-to-enter ≤0.05 and probability-of-F-to-remove ≥0.1. Statistical comparisons between different experimental groups were made by one-way analysis of variance followed by least significant difference (LSD) post hoc test.
Results
P70 S6 Kinase Immunoreactivity in Homogenates of AD and Control Brains by Western Blots
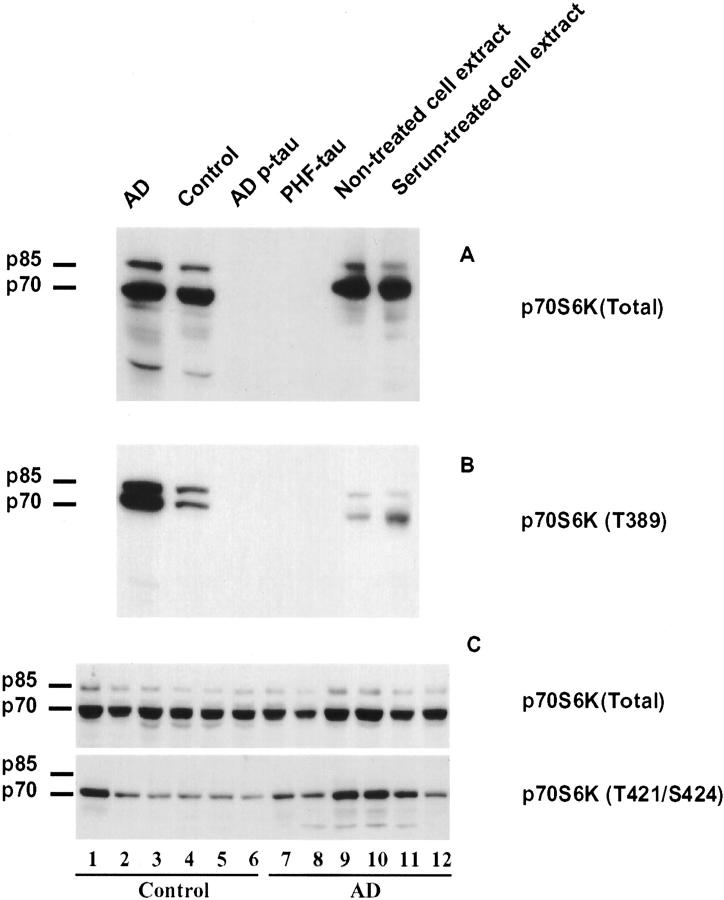
Antibody specificity was checked by Western blots of samples from AD and control homogenates, NIH-3T3 cell extracts, purified cytosolic abnormally hyperphosphorylated tau (AD p-tau) and PHF-tau. Antibody to total p70 S6 kinase (p70 S6K) showed two major positive bands corresponding to p70/p85 S6 kinases in samples from AD and control homogenates, and extracts of non-treated and serum-treated NIH-3T3 cells. The p70 S6 kinase band was much heavier than the p85 S6 kinase band. No bands were detected in lanes loaded with AD p-tau and PHF-tau (Figure 1A) ▶ . A minor band at about 40 kd, which is probably a degradative product of p70/p85 S6 kinase, was also found in AD and control homogenates. The antibody against p70 S6 kinase phosphorylated at T389, p70 S6K (T389), showed two heavy bands corresponding to phosphorylated p70 S6 and p85 S6 kinases in AD and relatively less intense bands in control homogenate (Figure 1B) ▶ . In non-treated and serum-treated NIH-3T3 cell extracts, p70 S6K showed a stronger band at about 70 kd than at 85 kd (Figure 1B) ▶ . After serum treatment, only the band at 70 kd showed an increase in signal intensity. mAb PHF-1 was used to confirm AD and control cases, AD p-tau and PHF-tau (data not shown).
Figure 1.
Characterization of antibodies to p70 S6 kinase and detection of p70 S6 kinase immunoreactivity in human brain homogenates by Western blots. Blots of homogenates from AD and control brains (12.5 μg protein/lane), AD p-tau (2 μg protein/lane), PHF-tau (2 μg protein/lane), and extracts from non-treated and serum-treated NIH-3T3 cells (negative and positive controls, respectively) were developed with antibodies to total p70 S6 kinase, p70 S6 kinase phosphorylated at either T389 or T421/S424. The p70 S6 kinase antibodies consistently identified p70/p85 kd S6 kinase in homogenates and NIH-3T3 cell lysates. None of the p70 S6 kinase antibodies showed cross-reaction with either AD p-tau or PHF-tau (A and B). No difference between AD and control cases was observed with total p70 S6 kinase (p70 S6K) whereas antibody to the p70 S6 kinase T421/S424 showed increase in most AD cases (C).
Levels of total p70 S6 kinase and phosphorylated p70 S6 kinase were compared in 6 control and 6 AD cases by Western blots. The antibody to p70 S6 kinase phosphorylated at T421/S424, p70 S6K (T421/S424), showed a strong single band at 70 kd and no detectable band at 85 kd (Figure 1C) ▶ . No significant difference was observed in total p70 S6 kinase (Figure 1C ▶ , top), whereas most AD cases showed higher phosphorylated p70 S6 kinase, especially at T421/S424 immunoreactivity than did controls (Figure 1C) ▶ .
Topography of the Increase in Active Forms of p70 S6 Kinase (T389) and p70 S6 Kinase (T421/S424) Coincides with the Progression of Alzheimer Neurofibrillary Changes
The topography of activated p70 S6 kinases (T389 and T421/S424) was investigated in the transentorhinal/entorhinal area, the hippocampal CA1, and layer III pyramidal neurons of the temporal cortex in brains showing different stages of AD neurofibrillary pathology. In normal controls, no immunostaining was found with antibodies to either of the two activated p70 S6 kinases (Figures 2a and 3a) ▶ . In accordance with the idea that the earliest neurofibrillary changes are initiated and predominate in the transentorhinal stage (stage I/II), we found a few neurons in the transentorhinal region containing punctate-like structures or tangle-like inclusions that were positive for antibodies to p70 S6 kinases (T389 and T421/S424). At this stage, other brain regions were still negative (Figures 2b and 3b) ▶ . In the moderate limbic stage (stage III-IV), an increased number of immunoreactive neurons containing various sizes of granules and tangle-like inclusions labeled by antibodies to p70 S6 kinases (T389 and T421/S424) were found in the Pre-α pyramidal neurons of the entorhinal region and hippocampal CA1 region (Figures 2c and 3c) ▶ . In the isocortical stages (stage V/VI), robust and widespread staining of neurons containing tangle-like inclusions and granules positive for antibodies to p70 S6 kinases (T389 and T421/S424) was observed in the entorhinal cortex, the hippocampal complex, and the temporal cortex (Figures 2d and 3d) ▶ . There was no significant gliosis in the tissues used including stage 0 and stages I to V.
Figure 2.
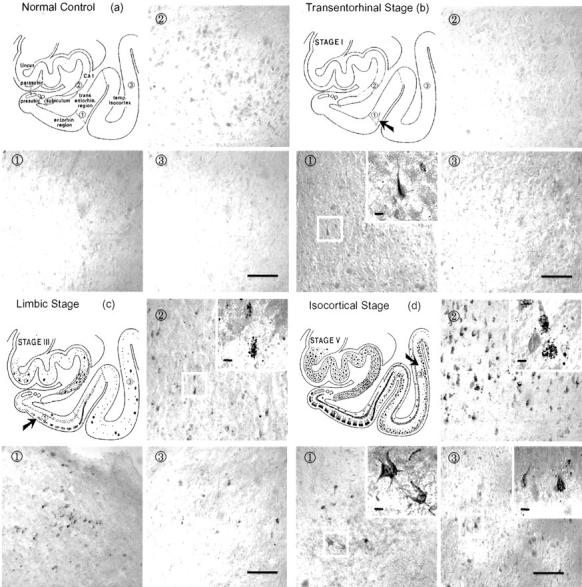
Immunohistochemical staining with antibody to activated p70 S6 kinase (T389) in brains with different degrees of neurofibrillary degeneration according to Braak’s staging criteria. The normal control that does not have any neurofibrillary involvement did not reveal any immunostaining (a). The early transentorhinal stage (I-II) that is characterized by mild neurofibrillary pathology in the transentorhinal region showed a few immunopositive neurons (b). The moderate limbic stage (III-IV) which is marked by a moderate involvement of neurofibrillary pathology in the entorhinal region, and the involvement of a few or many CA1 cells in the hippocampal region and temporal lobes showed immunostaining in several tangle-bearing neurons (c). The late isocortical stage (V-VI) which is characterized by the involvement of severe neurofibrillary pathology in the entorhinal cortex, the hippocampus, and isocortex showed the most robust immunostaining of neurons (d). Following the sequence of neurofibrillary degeneration from normal control to isocortical stage (V/VI), antibody to p70 S6 kinase phosphorylated at T389 showed progressively increased numbers of tangle-like inclusions and granular structures. Area 1 shows transentorhinal region in a and b or entorhinal region in c and d. Areas 2 and 3 show hippocampal CA1 and temporal isocortex, respectively in a, b, c and d. Bars in 3, 100 μm; bars in insets, 10 μm.
Distribution of the Active Forms of p70 S6 Kinase (T389) and p70 S6 Kinase (T421/S424) Coincides with PHF-Tau in Neurons
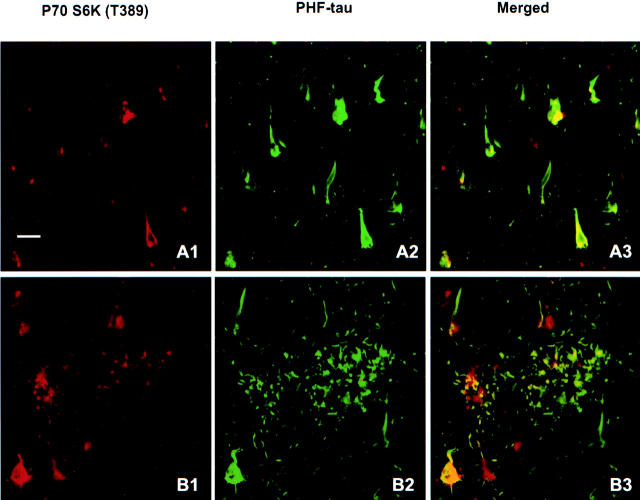
Double-immunofluorescent staining was carried out with antibodies to p70 S6 kinase (T389) (Figure 4) ▶ or p70 S6 kinase (T421/S424) (data not shown) and mAb AT8 to PHF-tau in sections from brains at Braak stages III, IV, and V. PHF-tau immunoreactivity in AD brain was restricted to neurons affected by NFT and accumulated in dystrophic neurites in neuritic plaques (Figure 4) ▶ . In most neurons, antibodies to p70 S6 kinase (T389) (Figure 4) ▶ or p70 S6 kinase (T421/S424) (data not shown) were found to partially co-localize with PHF-tau labeled by mAb AT8.
Figure 4.
Immunohistochemical co-localization of active p70 S6 kinase (T389) with PHF-tau (labeled by AT8). Active p70 S6 kinase (red) was partially or fully co-localized with AT8-labeled PHF-tau (green) in neuronal cytoplasm (A1, A2, and A3), and in a few dystrophic neurites (B1, B2, and B3). Bar, 20 μm.
The immunohistochemical staining with antibodies to p70 S6 kinase (T389) (data not shown) or p70 S6 kinase (T421/S424) (Figure 5) ▶ was further examined in neurons with different extent of PHF-tau as labeled by AT8. Four patterns of neurons could be distinguished in brains of stages III, IV, and V. Pattern 1 neurons were AT8-negative but showed granular staining for p70 S6 kinase (T389) (not shown) and for p70 S6 kinase (T421/S424) in cytoplasm (Figure 5, A1, A2, and A3) ▶ . Pattern 2 neurons showed aggregates of AT8/PHF-tau as granules in neuronal soma and neuropil threads. Only a few of the tau granules were also positive for p70 S6 kinase (T389) (not shown) or p70 S6 kinase (T421/S424) (Figure 5, B1, B2, and B3) ▶ . Pattern 3 neurons showed some filamentous structures in neuronal soma positive for p70 S6 kinase (T389) (not shown) or p70 S6 kinase (T421/S424) that overlapped with AT8/PHF-tau. In addition, granular staining of the kinase that was not associated with AT8 labeling was seen in the neuronal cytoplasm (Figure 5, C1, C2, and C3) ▶ . Pattern 4 neurons showed classic tangles labeled by AT8 that overlapped with a relatively weaker filament-like immunostaining of p70 S6 kinase (T389) (not shown) or p70 S6 kinase (T421/S424) (Figure 5, D1, D2, and D3) ▶ .
Figure 5.
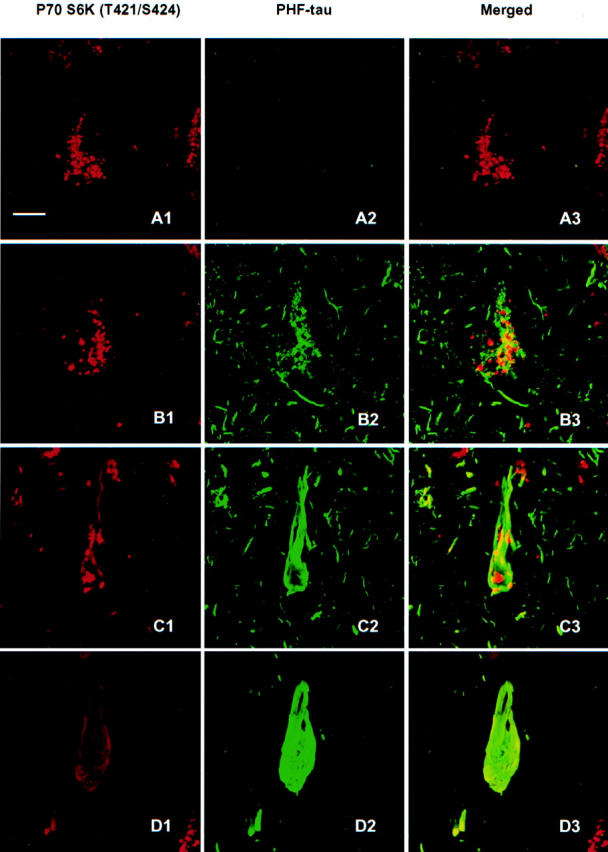
Immunoreactivity of active P70 S6 kinase (T421/S424) in neurons with different degrees of AT8-labeled PHF-tau involvement. In the normal-looking neurons, only active p70 S6 kinase was positive in granular form (A1, A2, and A3). In the pre-tangle neurons, some of the granular stainings of active p70 S6 kinase was partially overlapped with dotted AT8 labeled PHF-tau (B1, B2, and B3). In classic tangle bearing neurons, the granular staining of active p70 S6 kinase congregated, and some particle staining distributed along with the AT8-positive filamentous structures (C1, C2, and C3). At the advanced stage of tangle neurons, no dot-like active p70 S6 kinase staining could be seen, except for the faint filamentous structures partially overlapping with AT8 positive tangles (D1, D2, and D3). Bars, 10 μm.
Levels of p70 S6 Kinase and Tau Protein in AD and Control Brains as Determined by ELISA
To further understand the relationship between activated p70 S6 kinase and tau in AD, the levels of p70 S6 kinase and tau were determined and compared in homogenates of 22 AD and 13 control medial-temporal cortices by ELISA. The active forms of p70 S6 kinases (T389 and T421/S424) as well as total tau (labeled by R134 days) and PHF-tau (labeled by PHF-1 or AT8) were significantly increased in AD as compared to control cases (Figure 6, A and B) ▶ . The levels of total p70 S6 kinase and tau protein labeled by Tau-1 in AD brain were not significantly changed.
Figure 6.
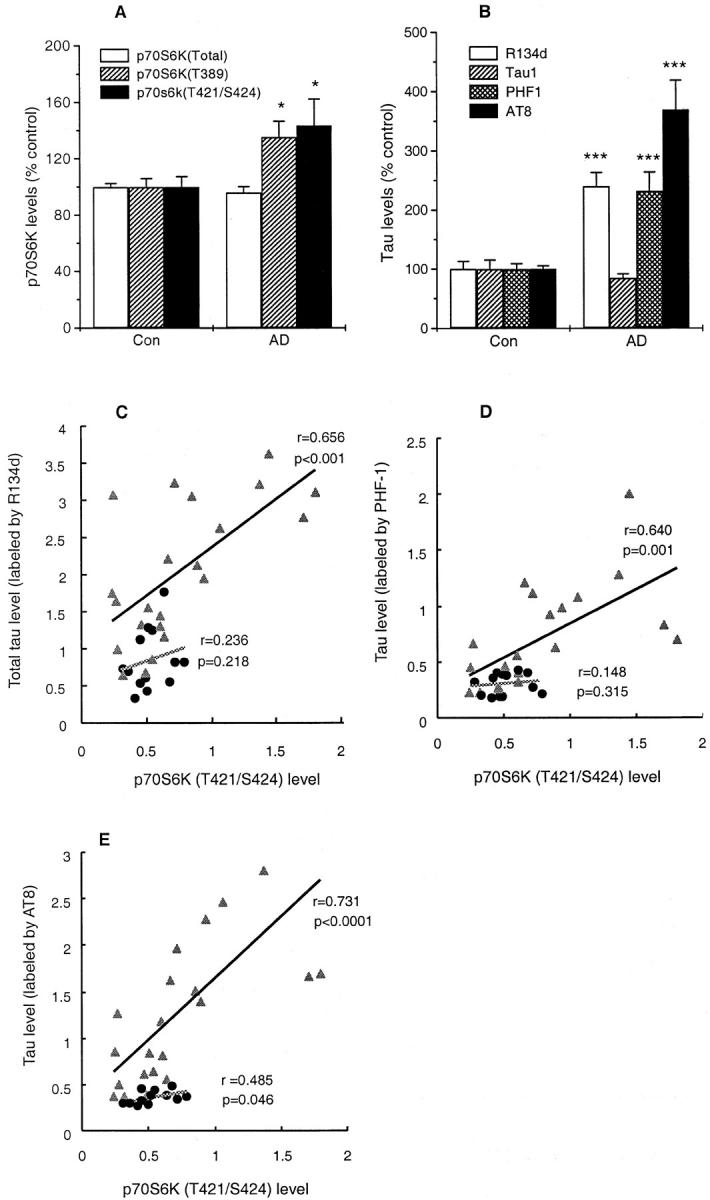
Levels of p70 S6 kinase and tau, and their correlations in human brain homogenates. Levels of active p70 S6 kinases (T389, T421/S424), total tau (labeled by R134 days), and PHF-tau (labeled by PHF-1 or AT8) were significantly increased in AD as compared with control (A and B). Levels of total p70 S6 kinase and normal tau (labeled by Tau-1) did not differ significantly between AD and controls (A and B). A and B: Mean ± SEM of 13 control and 22 AD cases. * indicates P < 0.05, and *** indicates P ≤ 0.001. The Pearson correlation analyses showed that levels of total p70 S6 kinase and active p70 S6 kinase (T421/S424) rather than active p70 S6 kinase (T389) showed significant correlations with total tau and PHF-tau (labeled by PHF-1 or AT8) in AD homogenates (C, D, and E; Table 4 ▶ ). Immunoreactivities of p70 S6 kinase and tau are presented as percentage of the OD values of control cases per 2 μg protein. ▴, AD cases and •, control individuals.
Correlation and regression analyses were carried out to investigate the relationship between the levels of p70 S6 kinase and tau protein. In AD cases, the levels of total p70 S6 kinase showed significant correlations with total tau labeled by R134 (P = 0.015) and PHF-tau labeled either by PHF-1 (P = 0.004) or AT8 (P = 0.001) (Table 4) ▶ . Similarly, levels of p70 S6 kinase (T421/S424) showed significant correlations with total tau (P = 0.00046) and PHF-tau labeled by PHF-1 (P = 0.001) or AT8 (P = 0.000057) (Table 4) ▶ . The level of p70 S6 kinase (T389) which, though increased in AD (Figure 6A) ▶ , did not show a significant correlation with total tau and PHF-tau for the AD cases (Table 4) ▶ . However, in the control cases, the level of p70 S6 kinase (T389) did show significant correlations with both PHF-1/PHF-tau (P < 0.028) and AT8/PHF-tau (P < 0.026), but not total tau. In contrast, the level of p70 S6 kinase (T421/S424) in control showed a significant correlation with AT8/PHF-tau (P < 0.046) but not PHF-1/PHF-tau (P < 0.315) (Table 4) ▶ .
Table 4.
Correlation and Regression Analyses for the Relationship Between Levels of p70 S6 Kinase and Tau Protein
| Tau protein | Groups | P70 S6 Kinase | Pearson correlation | Regression analysis | ||
|---|---|---|---|---|---|---|
| Correlation coefficient | P value | Variable entered | R2 | |||
| R134d/Total tau | AD | P70 S6K | 0.463 | 0.015* | P70 S6K (T421/S424) | 0.431 |
| P70 S6K(T389) | 0.159 | 0.240 | ||||
| P70 S6K(T421/S424) | 0.656 | 0.00046*** | ||||
| Control | P70 S6K | −0.366 | 0.109 | |||
| P70 S6K(T389) | 0.352 | 0.119 | ||||
| P70 S6K(T421/S424) | 0.236 | 0.218 | ||||
| PHF-1/PHF-tau | AD | P70 S6K | 0.550 | 0.004** | P70 S6K (T421/S424) | 0.409 |
| P70 S6K(T389) | 0.111 | 0.312 | ||||
| P70 S6K(T421/S424) | 0.640 | 0.001*** | ||||
| Control | P70 S6K | −0.461 | 0.056 | |||
| P70 S6K(T389) | 0.543 | 0.028* | ||||
| P70 S6K(T421/S424) | 0.148 | 0.315 | ||||
| AT8/PHF-tau | AD | P70 S6K | 0.632 | 0.001*** | P70 S6K(T421/S424) | 0.534 |
| P70 S6K(T389) | 0.242 | 0.139 | ||||
| P70 S6K(T421/S424) | 0.731 | 0.000057*** | ||||
| Control | P70 S6K | −0.360 | 0.113 | |||
| P70 S6K(T389) | 0.550 | 0.026* | ||||
| P70 S6K(T421/S424) | 0.485 | 0.046* | ||||
*P ≤0.05,
**P ≤0.01,
***P <0.001 vs. tau protein levels (labeled by R134d, PHF-1, and AT8)
Method for variables entered/removed: stepwise (criteria: probability-of-F-to-enter ≤0.05, probability-of-F-to-remove ≥0.10). The presented coefficient of determination, R2, measures the closeness of fit of the regression equation.
Step-wise regression analysis only showed dependency between levels of p70 S6 kinase (T421/S424), and total tau labeled by R134 and PHF-tau by PHF-1/AT8 for AD cases under the variable entered/removed criteria as described in Methods (Figure 6C, D, and E ▶ ; Table 4 ▶ ). There was no significant correlation between total p70 S6 kinase/p70 S6 kinase phosphorylated (T389) and total tau or PHF-tau both in AD and control brains (Table 4) ▶ .
P70 S6 Kinase, Ribosomal Protein S6, and Markers of the Proteolytic System
To clarify the origin of the punctate structures found positive for p70 S6 kinase, we performed immunohistochemistry with markers of endosomes (rab5), lysosomes (lamp-1), endoplasmic reticulum (Grp78, Bip), and ubiquitin-proteasomal structures (ubiquitin). Patterns 1 and 2 (Figure 5) ▶ neurons positive for p70 S6 kinase showed no obvious overlap between p70 S6 kinase (T421/S424) and lamp-1 immunoreactivity (Figure 7A, A1, A2, and A3) ▶ . Pattern 3 neurons only showed partial co-localization of p70 S6 kinase (T421/S424) with lamp-1 (Figure 7A, B1, B2, and B3) ▶ . This could be seen clearly in 12 serial optical sections (1.5-μm interval) obtained from the same neuron as Figure 7A, B1, B2, and B3 ▶ , and Figure 7B, 1 to 12 ▶ . Similar results could be observed with rab5, ubiquitin and Grp78 (Bip) (data not shown). AT8/PHF-tau-stained particles were partially co-localized with ubiquitin (data not shown).
Figure 7.
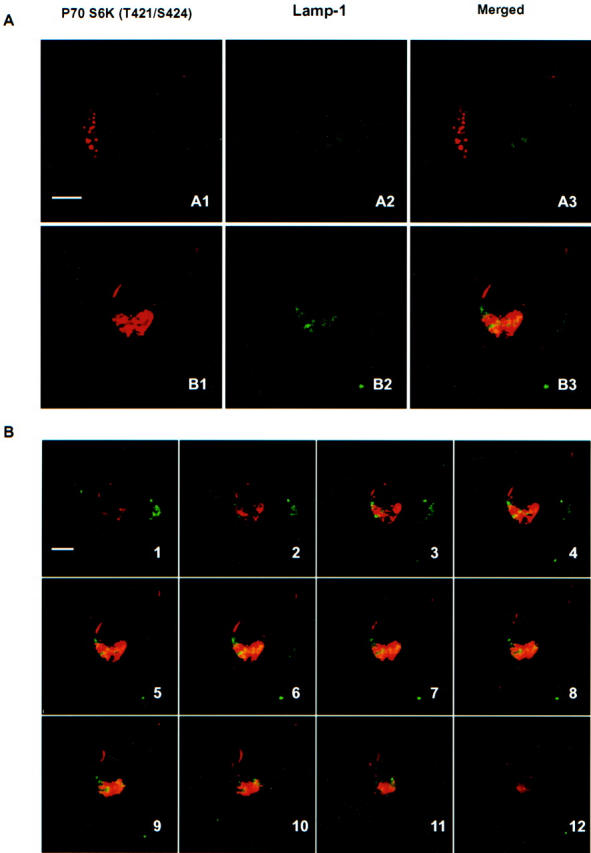
Co-localization of p70 S6 kinase (T421/S424) and lysosomes (lamp-1). In Patterns 1 and 2 neurons (see Figure 5 ▶ ), there was no obvious overlap between p70 S6 kinase (T421/S424) and lysosomes (A1, A2, and A3 of panel A). In Pattern 3 neurons, partial co-localization with lamp-1 could be detected in p70 S6 kinase positive structures (B1, B2, and B3 of panel A). Twelve serial optical sections (1.5-μm interval) were obtained from the same neuron as B1, B2, and B3 of panel A by confocal microscope. Bars, 10 μm.
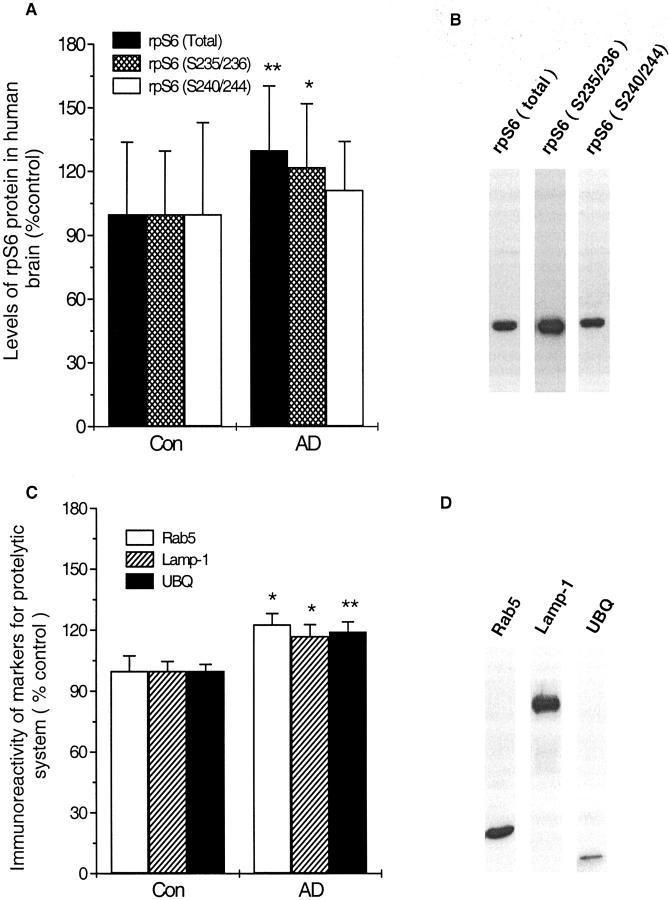
Levels of rpS6 (total), rpS6 (S235/236 and S240/244), and markers for the endosomal-lysosomal and ubiquitin-proteasomal systems were measured in AD and control cases by ELISA. Both total rpS6 and phosphorylated rpS6 at S235/236 but not phosphorylated rpS6 (S240/244) were significantly increased in AD brains (panel A of Figure 8 ▶ ). Levels of rab5, lamp-1, and ubiquitin all showed a significant increase in AD brain as compared with controls (Figure 8B) ▶ .
Figure 8.
Levels of immunoreactivity of ribosomal protein S6 (rpS6) and markers to proteolytic system in human brain homogenates. The immunoreactivities of both rpS6 (total) and phosphorylated rpS6 (S235/236), but not rpS6 (S240/244) were significantly increased in AD brains (A). Significant increase was also seen with antibodies to Rab5, Lamp-1 and ubiquitin in AD homogenates as compared with controls (B). The specificity of the antibodies was tested by Western blots, showing only one clear band corresponding to respective protein (B and D). Results represent the mean ± SEM of 13 control and 22 AD cases. * P < 0.05 and ** P < 0.01 compared with control group.
Effects of p70 S6 Kinase Phosphorylation/Activation on Levels and Phosphorylation of Tau in SH-SY5Y Neuroblastoma Cells and Rat Brain Primary Cultured Neurons
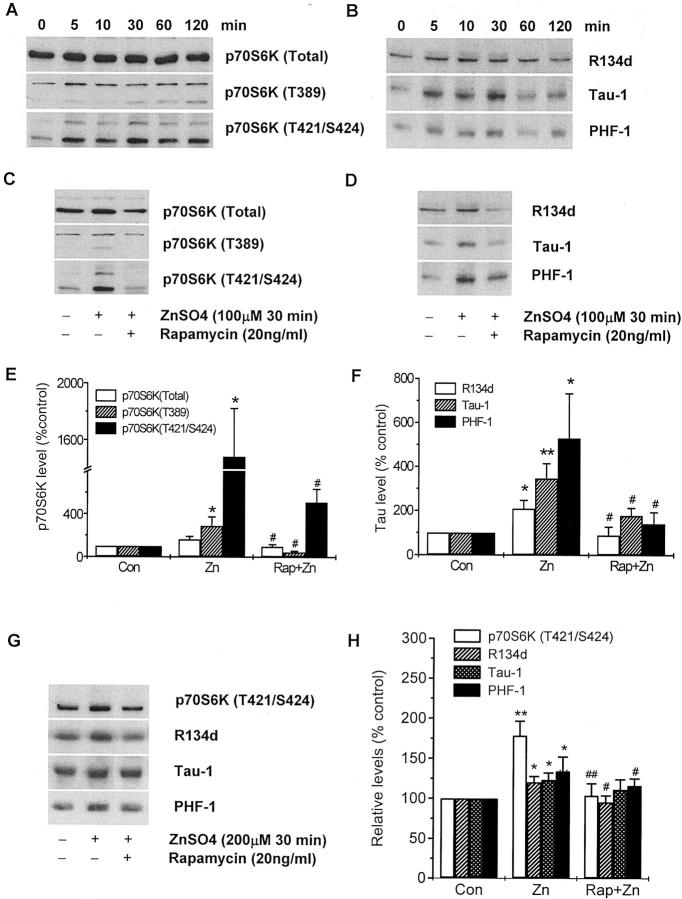
The time-dependent expression of total p70 S6 kinase, the phosphorylation of p70 S6 kinase at T389 and T421/S424 (Figure 9A) ▶ and increased levels of total tau (R134), normal tau (Tau-1), and hyperphosphorylated tau (PHF-1) (Figure 9B) ▶ were studied in SH-SY5Y cells following treatment with 100 μmol/L zinc sulfate. The level of total p70 S6 kinase did not show obvious alterations following zinc sulfate treatment. The level of p70 S6 kinase (T389) was increased at 5 minutes and remained elevated up to 120 minutes posttreatment. The level of p70 S6 kinase (T421/S424) showed an instant and dramatic increase at 5 minutes after zinc sulfate treatment and reached a maximal level at 30 minutes. Total tau protein (R134), normal tau (Tau-1), and hyperphosphorylated tau (PHF-1) showed an instant dramatic increase at 5 minutes posttreatment, a maximal level at 30 minutes, followed by a decrease. Pretreatment of cells with 20 ng/ml rapamycin for 1 hour inhibited the 100-μmol/L zinc sulfate-induced increase in levels of phosphorylated p70 S6 kinase, as well as levels of total, normal and hyperphosphorylated tau at 30 minutes (Figure 9, C–F) ▶ .
Figure 9.
The phosphorylation of p70 S6 kinase is associated with the accumulation and phosphorylation of tau protein. SH-SY5Y cells were cultured in DMEM/F12 (1:1) containing 5% fetal bovine serum (FBS) for 4 days, and then in media containing 0.5% FBS for 2 days. The time-dependent expression and phosphorylation of p70 S6 kinase (A) and tau (B) were detected by Western blots in SH-SY5Y cells treated with 100 μmol/L zinc sulfate. The phosphorylated p70 S6 kinase and tau protein showed instant increase after 5 minutes exposure with zinc sulfate, and the increase reached a significant high level at 30 minutes of treatment. Pretreatment of SH-SY5Y cells with 20 ng/ml rapamycin for 1 hour inhibited the effects of zinc sulfate on the phosphorylation of p70 S6 kinase (C) and tau phosphorylation and accumulation (D). Exposure of rat brain primary cultured neurons with 200 μmol/L zinc sulfate for 30 minutes showed significant increase of phosphorylated p70 S6 kinase at T421/S424, total tau (R134), normal tau (Tau-1), and phosphorylated tau (PHF-1). Pretreatment of the primary cultures with rapamycin for 1 hour resulted in decreased levels of phosphorylated p70 S6 kinase and tau induced by zinc sulfate treatment (G and H). The blots are representative from three or four experiments. Results in E, F, and H were expressed as percentage value relative to non-treated cells. E, F, and H: Means ± SEM of at least three independent experiments of C and D. Statistical analyses were made using analysis of variance with LSD post hoc test. * P < 0.05 and ** P < 0.01 compared with untreated cells; # P < 0.05 and ## P < 0.01 compared with zinc sulfate (100 μmol/L) 30-minute-treated group.
Exposures of primary-cultured cortical neurons from rat brain at embryonic 16- to 18-days-old with zinc 200 μmol/L for 30 minutes significantly increased levels of phosphorylated p70 S6 kinase at T421/S424, total tau (R134), normal tau (Tau-1), and phosphorylated tau (PHF-1). Pretreatment of the primary cultured neurons with rapamycin for 1 hour resulted in attenuation of phosphorylated p70 S6 kinase at T421/S424, total tau (R134) and phosphorylated tau (PHF-1), but not normal tau (Tau-1) (Figure 9, G and H) ▶ .
Discussion
P70 S6 kinase plays a critical role in regulating the translational activity of the cell. 13 To understand the pathogenesis of PHF-tau accumulation in AD, we explored in the present study the relationship between p70 S6 kinase and the level and the abnormal hyperphosphorylation of tau. We have found: 1) that the distribution of activated p70 S6 kinases (T389, T421/S424) in human brain coincides with the progression of neurofibrillary degeneration according to Braak’s staging; 2) that the activated p70 S6 kinases (T389, T421/S424) appear to accumulate in neurons before developing tangles; 3) that the increases of total tau and PHF-tau level significantly correspond with and are dependent on the immunoreactivity of p70 S6 kinase phosphorylated at T421/S424; 4) that although the levels of activated p70 S6 kinase phosphorylated at both T389 and T421/S424 are increased significantly in AD as compared to control, PHF-tau shows dependent correlations only with the level of activated p70 S6 kinase (T421/S424); 5) the levels of total rpS6 and phosphorylated S6 at S235/236 are elevated; 6) that zinc-induced phosphorylation of p70 S6 kinase, in particular at T421/S424 sites, correlates with the levels of total, normal, and hyperphosphorylated tau species in SH-SY5Y cells; and 7) that zinc sulfate induces significant increase for both p70 S6 kinase at T421/S424 and total tau in rat brain primary-cultured neurons.
PHF-tau formation has been suggested to result from an altered balance between the activities of tau protein kinases and protein phosphatases. 2,39 A number of proline-directed kinases have been implicated in the hyperphosphorylation of PHF-tau, including cyclin-dependent kinase 5 (cdk5), glycogen synthase kinase-3 (GSK-3), cell division cycle 2 (cdc2) kinase, and MAPK. 40-44 Despite PHF-tau accumulation in neurons affected by NFTs, 1-2 a considerable amount of normal tau remains in AD brain. 7 The total tau mRNA level is normal in AD brain. 45 Yasojima et al 46 have reported that in areas of AD brain with a heavy load of NFTs, the exon-10-containing isoforms of tau mRNA are up-regulated. In rat brain, an increase in tau protein level has been shown to occur independently of changes in tau mRNA expression following amyloid-β injection. 47 In contrast, increased tau protein levels were shown to accompany a transient up-regulation of tau mRNA in the early phase of chloroquine-induced myopathy in muscle. 48,49
Although more lysosomes and endosomes are produced in AD brain, 50 and other protein degradation systems such as ubiquitin-proteasomal system are also involved, 51 these proteolytic systems might not be sufficient to degrade or modify over-produced and subsequently over-phosphorylated PHF-tau. 52 The present study showed that activated p70 S6 kinase accumulated in neurons before developing tangles (Pattern 1 and 2 neurons) in granular forms, which were reminiscent of lysosome structures. So we first thought that neurons at a pretangle stage might attempt to mobilize the endosomal-lysosomal system to counteract incoming accumulated tau protein induced by up-regulated p70 S6 kinase. However, double-immunostaining showed only partial co-localization of p70 S6 kinase-positive particles with lamp-1, Rab5, or with ubiquitin in Pattern 3 neurons. Similarly, AT8/PHF-tau-positive particles did not totally overlap with ubiquitin staining. These findings suggest that to some extent accumulated phosphorylated p70 S6 kinase and PHF-tau might not be degraded and modified in either the lysosome or ubiquitin-proteasomal systems. It is likely that Pattern 1 neurons simply represented the aggregates of p70 S6 kinase phosphorylated at T389 or T421/S424.
The correlation of PHF-tau and total tau levels with activated p70 S6 kinase (T421/S424) suggested that T421/S424 phosphorylation and activation of p70 S6 kinase might increase translational capacity and tau production in AD. This is further strengthened by the increase of total ribosomal protein S6 encoded by one of TOP mRNAs and phosphorylated rpS6 at S235/236 in AD brain, since as a direct substrate of p70 S6 kinase, rpS6 phosphorylation at S235/236 implies p70 S6 kinase activation. 19 Treatment of SH-SY5Y cells or rat brain primary-cultured neurons with zinc sulfate was shown to induce phosphorylation and activation of p70 S6 kinase and to also increase the synthesis and phosphorylation of tau protein. This suggests a potential cause-effect relationship between p70 S6 kinase and tau translation. A continuous synthesis of normal tau induced by up-regulated p70 S6 kinase in AD brain might act to compensate for the biologically inactive PHF-tau abnormally hyperphosphorylated by kinases such as cdk5, GSK-3, cdc2, PKA, CaMKII, and MAPK. 40-42,53-56
Accumulation of a number of other proteins, such as cdc2/cyclinB, cdc25B tyrosine phosphatase, cdk5, Ki-67, nucleolin, neurofilament, and cyclin D has also been reported in AD brain. 44,57-63 Most of these proteins are associated with the cell cycle that is known to be also regulated by p70 S6 kinase. 20 In addition, p70 S6 kinase plays an important role in protein synthesis, and is likely involved in production of a large number of proteins needed during re-entering of the cell cycle for differentiated mature neurons under environmental stimuli, or during neuroblastma cell mitosis. Therefore, it is unlikely that the accumulated phosphorylated p70 S6 kinase is just a derivative effect of accumulation of other cell-cycle-associated proteins, because p70 S6 kinase activation induced by zinc ions was found to generate accumulation and phosphorylation of tau protein in SH-SY5Y cells. It is possible that the phosphorylated p70 S6 kinase is functional in the pretangle neurons and contributes also to the accumulation of cell-cycle-associated proteins in AD brain.
It is thought that the activation of p70 S6 kinase by its phosphorylation at T421/S424 sites could be induced by many mitogenic stimuli through the MAPK pathway and then by a kinase different from phosphoinositide-dependent kinase 1 in the PI3K pathway. 14,17 Consequently, the MAPK and PI3K pathways might also contribute to abnormal accumulation of the other proteins mentioned above in response to exogenous stimuli, such as zinc ions, β-amyloid and a range of extracellular trophic signals. 24,64,65 Although neurofibrillary pathology was found in double-transgenic mice with both tau and APP mutations, 66 and more severe in tau transgenic mice following injection of β-amyloid1–42 fibrils, 67 the interaction between β-amyloid and tau pathologies is not quite clear, because such experimental mouse models do not necessarily support the hypothesis that a similar interaction occurs in AD. AD has polyetiology; the vast number of AD cases is sporadic. Also, the incidence of AD is affected by many other factors including age, head trauma, gender, education, and environmental exposures. 68 The activation of MEK1/2, ERK1/2, JNK, p38, cdc2, and p70 S6 kinase is seen to occur in brains with neurofibrillary stages I-III, 69,70 which did not show deposition of β-amyloid. Thus, it is possible that other factors such as elevated zinc ions and other stimuli may induce the sequential activation of MEK1/2, ERK1/2, and p70 S6 kinase at this early stage of neurofibrillary degeneration. 71 It cannot be ruled out that in tau transgenic mice the over-loading of β-amyloid results in a stress to adjacent neurons, and subsequent activation of JNK that may phosphorylate tau to be a better substrate for other kinases such as cdk5, GSK-3, and MAPK.
Protein-serine/threonine phosphatase 2A (PP-2A) is thought to be the main candidate phosphatase involved in neurofibrillary degeneration in AD brain. 54,72-75 The co-immunoprecipitation of p70 S6 kinase and PP-2A from microtubules, suggests that like PP-2A, p70 S6 kinase is associated with microtubules. 76,77 PP-2A was previously shown to regulate the activity of p70 S6 kinase. 77,78 In the current study, regression analysis showed a selective dependency of PHF-tau on p70 S6 kinase phosphorylated at T421/S424. Consistent with this, confocal microscopy data showed the co-localization of immunohistochemical staining for both AT8/PHF-tau and activated p70 S6 kinase. Thus, it is likely that reduced PP-2A activity might not only induce a compromised PHF-tau dephosphorylation, but may also cause PHF-tau accumulation as a result of up-regulated p70 S6 kinase activation.
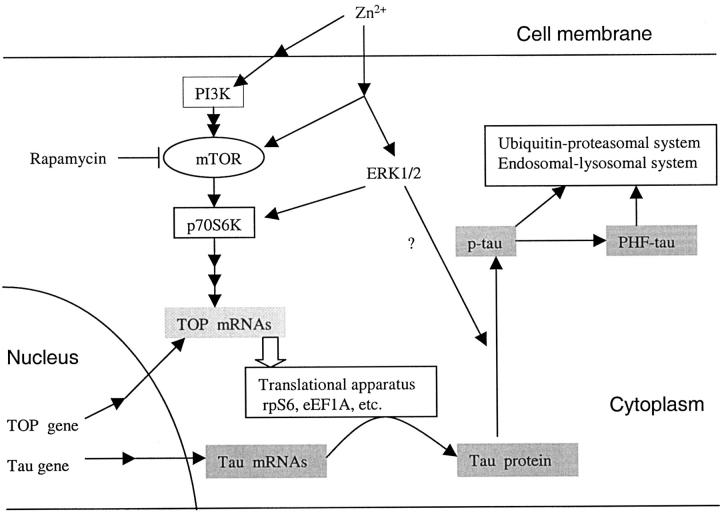
As hypothesized in Figure 10 ▶ , the production and phosphorylation of tau protein is regulated by p70 S6 kinase. The distribution of activated p70 S6 kinase coincided with the progression of neurofibrillary degeneration. Accumulation of activated p70 S6 kinase appeared to precede the accumulation of abnormally hyperphosphorylated tau. The levels of p70 S6 kinase (T389) and p70 S6 kinase (T421/S424) but not total p70 S6 kinase were increased in AD. The levels of total p70 S6 kinase and activated p70 S6 kinase (T421/424) significantly correlated with the levels of total tau and PHF-tau in AD brain. The accumulation of total tau and PHF-tau was selectively dependent on the activated p70 S6 kinase (T421/S424). Zinc-induced phosphorylation and activation of p70 S6 kinase, and the corresponding increased synthesis and phosphorylation of tau protein are in support of the postulate that p70 S6 kinase activation is involved in NFT formation by up-regulating tau translation in AD.
Figure 10.
Hypothetical scheme showing the effects of zinc-induced p70 S6 kinase activation by PI3K and MAPK pathways on the accumulation of tau protein. Zinc induces p70 S6 kinase activation, and then enhances the translation of TOP mRNA coding the translation apparatus, and results in the increase of translational capacity of tau protein. Even though the proteolytic system was also involved, it appears to be insufficient relative to the production for the degradation of tau
Supplementary Material
Figure 3.
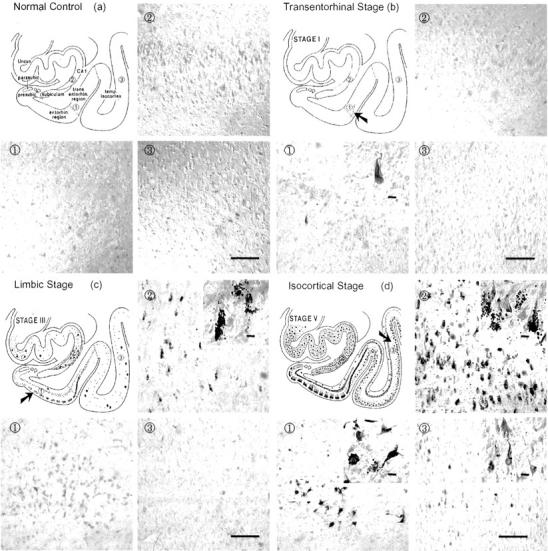
Immunohistochemical staining with antibody to activated p70 S6 kinase (T421/S424) in brains with different degrees of neurofibrillary degeneration according to Braak’s staging criteria. 28 Following the sequence of neurofibrillary degeneration (a to d, same as in Figure 2 ▶ ) from normal control to Stage V/VI, antibody to p70 S6 kinase (T421/S424) showed progressively increased numbers of tangle-like inclusions and granular structures. Bars in 3, 100 μm; bars in insets, 10 μm.
Acknowledgments
We thank Dr. Wen-Yong Huang for help with statistic analysis, and Drs. Angela Garcia Jimenez and Angel Cedazo-Minguez for help with Western blots.
Footnotes
Address reprint requests to Jin-Jing Pei, MD., Ph.D., Karolinska Institutet, Neurotec, Division of Experimental Geriatrics, KFC Plan 4, Novum, S-141 86, Huddinge, Sweden. E-mail: jin-jing.pei@neurotec.ki.sc.
Supported by Alzheimerfonden, Fredrik och Ingrid Thurings Stiftelse, Gamla Tjänarinnor Foundation, Gun och Bertil Stohnes Stiftelse, Karolinska Institutets Stiftelser, Tore Nilssons Stiftelse, Loo and Hans Ostermans Foundation, Svenskaläkaresällskapet, Åke Wibergs Stiftelse, Beijing Municipal Natural Science Foundation of China (grant number 7032013), the Deutsche Forschungsgemeinschaft, NIH grants AG08076 and AG19158, and an IIRG grant from the Alzheimer’s Association, Chicago, IL.
References
- 1.Grundke-Iqbal I, Iqbal K, Quinlan M, Tung Y-C, Zaide MS, Wisniewski HM: Microtubule-associated protein tau, a component of Alzheimer-paired helical filaments. J Biol Chem 1986, 261:6084-6089 [PubMed] [Google Scholar]
- 2.Grundke-Iqbal I, Iqbal K, Tung Y-C, Quinlan M, Wisniewski HM, Binder I: Abnormal phosphorylation of the microtubule-associated protein tau in Alzheimer cytoskeleton pathology. Proc Nat Acad Sci USA 1986, 83:4913-4917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braak H, Braak E, Grundke-Iqbal I, Iqbal K: Occurrence of neuropil threads in the senile human brain and in Alzheimer’s disease: a third location of paired helical filaments outside of neurofibrillary tangles and neuritic plaques. Neurosci Lett 1986, 165:351-355 [DOI] [PubMed] [Google Scholar]
- 4.Iqbal K, Grundke-Iqbal I, Zaidi T, Merz PA, Wen GY, Shaikh SS, Wisniewski HM, Alafuzoff I, Winblad B: Defective brain microtubule assembly in Alzheimer’s disease. Lancet 1986, 2:421-426 [DOI] [PubMed] [Google Scholar]
- 5.Iqbal K, Grundke-Iqbal I, Smith AJ, George L, Tung Y-C, Zaidi T: Identification and localization of a tau peptide to paired helical filaments of Alzheimer disease. Proc Natl Acad Sci USA 1989, 86:5646-5650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khatoon S, Grundke-Iqbal I, Iqbal K: Brain levels of microtubule-associated protein tau are elevated in Alzheimer’s disease: a radioimmuno-slot-blot assay for nanograms of the protein. J Neurochem 1992, 59:750-753 [DOI] [PubMed] [Google Scholar]
- 7.Khatoon S, Grundke-Iqbal I, Iqbal K: Levels of normal and abnormally phosphorylated tau in different cellular and regional compartments of Alzheimer disease and control brains. FEBS Lett 1994, 351:80-84 [DOI] [PubMed] [Google Scholar]
- 8.Alonso A del C, Zaidi T, Grundke-Iqbal I, Iqbal K: Role of abnormally hyperphosphorylated tau in the breakdown of microtubules in Alzheimer disease. Proc Natl Acad Sci USA 1994, 91:5562-5566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alonso AC, Grundke-Iqbal I, Iqbal K: Alzheimer’s disease hyperphosphorylated tau sequesters normal tau into tangles of filaments and disassembles microtubules. Nat Med 1996, 2:783-787 [DOI] [PubMed] [Google Scholar]
- 10.Grove JR, Banerjee P, Balasubramanyam A, Coffer PJ, Price DJ, Avruch J, Woodgett JR: Cloning and expression of two human p70 S6 kinase polypeptides differing only at their amino termini. Mol Cell Biol 1991, 11:5541-5550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reinhard C, Thomas G, Kozma SC: A single gene encodes two isoforms of the p70 S6 kinase: activation upon mitogenic stimulation. Proc Natl Acad Sci USA 1992, 89:4052-4056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reinhard C, Fernande A, Lamb NJ, Thomas G: Nuclear localization of p85s6k: functional requirement for entry into S phase. EMBO J 1994, 13:1557-1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dufner A, Thomas G: Ribosomal S6 kinase signaling and the control of translation. Exp Cell Res 1999, 253:100-109 [DOI] [PubMed] [Google Scholar]
- 14.Domin J, Waterfield MD: Using structure to define the function of phosphoinositide 3-kinase family members. FEBS Lett 1997, 410:91-95 [DOI] [PubMed] [Google Scholar]
- 15.Jeno P, Ballou LM, Novak-Hofer I, Thomas G: Identification and characterization of a mitogenic-activated S6 Kinase. Proc Natl Acad Sci USA 1988, 85:406-410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mukhopadhyay NK, Price DJ, Kyriakis JM, Pelech S, Sanghera J, Avruch J: An array of insulin-activated, proline-directed serine/threonine protein kinases phosphorylate the p70 S6 kinase. J Biol Chem 1992, 267:3325-3335 [PubMed] [Google Scholar]
- 17.Pullen N, Dennis PB, Andjelkovic M, Dufner A, Kozma SC, Hemmings BA, Thomas G: Phosphorylation and activation of p70s6k by PDK1. Science 1998, 279:707-710 [DOI] [PubMed] [Google Scholar]
- 18.Stewart MJ, Thomas G: Mitogenesis and protein synthesis: a role for ribosomal protein S6 phosphorylation? BioEssays 1994, 16:1-7 [DOI] [PubMed] [Google Scholar]
- 19.Flotow H, Thomas G: Substrate recognition determinants of the mitogen-activated 70K S6 kinase from rat liver. J Biol Chem 1992, 267:3074-3078 [PubMed] [Google Scholar]
- 20.Lane HA, Fernandez A, Lamb NJ, Thomas G: p70s6k function is essential for G1 progression. Nature 1993, 363:170-172 [DOI] [PubMed] [Google Scholar]
- 21.Berven LA, Crouch MF: Cellular function of p70S6K: a role in regulating cell motility. Immunol Cell Biol 2000, 78:447-451 [DOI] [PubMed] [Google Scholar]
- 22.Braak H, Braak E: Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol (Berl) 1991, 82:239-259 [DOI] [PubMed] [Google Scholar]
- 23.Bae GU, Seo DW, Kwon HK, Lee HY, Hong S, Lee ZW, Ha KS, Lee HW, Han JW: Hydrogen peroxide activates p70 (S6k) signaling pathway. J Biol Chem 1999, 274:32596-32602 [DOI] [PubMed] [Google Scholar]
- 24.Kim S, Jung Y, Kim D, Koh H, Chung J: Extracellular zinc activates p70 S6 kinase through the phosphatidylinositol 3-kinase signaling pathway. J Biol Chem 2000, 275:25979-25984 [DOI] [PubMed] [Google Scholar]
- 25.Tu VC, Bahl JJ, Chen QM: Signals of oxidant-induced cardiomyocyte hypertrophy: key activation of p70 S6 kinase-1 and phosphoinositide 3-kinase. J Pharmacol Exp Ther 2002, 300:1101-1110 [DOI] [PubMed] [Google Scholar]
- 26.Lynch CJ, Patson BJ, Goodman SA, Trapolsi D, Kimball SR: Zinc stimulates the activity of the insulin- and nutrient-regulated protein kinase mTOR. Am J Physiol Endocrinol Metab 2001, 281:E25-E34 [DOI] [PubMed] [Google Scholar]
- 27.Deibel MA, Ehmann WD, Markesbery WR: Copper, iron, and zinc imbalances in severely degenerated brain regions in Alzheimer’s disease: possible relation to oxidative stress. J Neurol Sci 1996, 143:137-142 [DOI] [PubMed] [Google Scholar]
- 28.Danscher G, Jensen KB, Frederickson CJ, Kemp K, Andreasen A, Juhl S, Stoltenberg M, Ravid R: Increased amount of zinc in the hippocampus and amygdala of Alzheimer’s diseased brains: a proton-induced X-ray emission spectroscopic analysis of cryostat sections from autopsy material. J Neurosci Methods 1997, 76:53-59 [DOI] [PubMed] [Google Scholar]
- 29.Cornett CR, Markesbery WR, Ehmann WD: Imbalances of trace elements related to oxidative damage in Alzheimer’s disease brain. Neurotoxicology 1998, 19:339-345 [PubMed] [Google Scholar]
- 30.Köpke E, Tung Y-C, Shaikh S, Alonso A del C, Iqbal K, Grundke-Iqbal I: Microtubule-associated protein tau: abnormal phosphorylation of a non-paired helical pool in Alzheimer disease. J Biol Chem 1993, 268:24374-24384 [PubMed] [Google Scholar]
- 31.Tatebayashi Y, Iqbal K, Grundke-Iqbal I: Dynamic regulation of expression and phosphorylation of tau by fibroblast growth factor-2 in neural progenitor cells from adult rat hippocampus. J Neurosci 1999, 9:5245-5254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Braak H, Braak E: Alzheimer’s disease affects limbic nuclei of the thalamus. Acta Neuropathol (Berl) 1991, 81:261-268 [DOI] [PubMed] [Google Scholar]
- 33.Braak H, Braak E, Ohm T, Bohl J: Silver impregnation of Alzheimer’s neurofibrillary changes counterstained for basophilic material and lipofuscin pigment. Stain Technol 1988, 63:197-200 [DOI] [PubMed] [Google Scholar]
- 34.Gallyas F: Silver staining of Alzheimer’s neurofibrillary changes by means of physical development. Acta Morphol Acad Sci Hung 1971, 19:1-8 [PubMed] [Google Scholar]
- 35.Braak E, Braak H, Mandelkow EM: A sequence of cytoskeleton changes related to the formation of neurofibrillary tangles and neuropil threads. Acta Neuropathol (Berl) 1994, 87:554-567 [DOI] [PubMed] [Google Scholar]
- 36.Campbell SK, Switzer RC, Martin TL: Alzheimer’s plaques and tangles: a controlled and enhanced silver-staining method. Soc Neurosci Abstr 1987, 13:H678 [Google Scholar]
- 37.Braak H, Braak E, Bohl J: Staging of Alzheimer-related cortical destruction. Eur Neurol 1993, 33:403-408 [DOI] [PubMed] [Google Scholar]
- 38.Bradford MM: A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976, 72:248-254 [DOI] [PubMed] [Google Scholar]
- 39.Iqbal K, Grundke-Iqbal I: Ubiquitination and abnormal phosphorylation of paired helical filaments in Alzheimer’s disease. Mol Neurobiol 1992, 5:399-410 [DOI] [PubMed] [Google Scholar]
- 40.Ishiguro K, Omori A, Sato K, Tomizawa K, Imahori K, Uchida T: A serine/threonine proline kinase activity is included in the tau protein kinase fraction forming a paired helical filament epitope. Neurosci Lett 1991, 128:195-198 [DOI] [PubMed] [Google Scholar]
- 41.Hanger DP, Hughes K, Woodgett JR, Brion JP, Anderton BH: Glycogen synthase kinase-3 induces Alzheimer’s disease-like phosphorylation of tau: generation of paired helical filament epitopes and neuronal localisation of the kinase. Neurosci Lett 1992, 147:58-62 [DOI] [PubMed] [Google Scholar]
- 42.Ledesma MD, Correas I, Avila J, Diaz-Nido J: Implication of brain cdc2 and MAP2 kinases in the phosphorylation of tau protein in Alzheimer’s disease. FEBS Lett 1992, 308:218-224 [DOI] [PubMed] [Google Scholar]
- 43.Sengupta A, Kabat J, Novak M, Wu Q, Grundke-Iqbal I, Iqbal K: Protein phosphorylation of tau at both Thr 231 and Ser 262 is required for maximal inhibition of its binding to microtubules. Arch Biochem Biophys 1998, 357:299-309 [DOI] [PubMed] [Google Scholar]
- 44.Pei J-J, Braak H, Gong C-X, Grundke-Iqbal I, Iqbal K, Winblad B, Cowburn RF: Up-regulation of cell division cycle (cdc) 2 kinase in neurons with early stage Alzheimer’s disease neurofibrillary degeneration. Acta Neuropathologica 2002, 104:369-376 [DOI] [PubMed] [Google Scholar]
- 45.Mah VH, Eskin TA, Kazee AM, Lapham L, Higgins GA: In situ hybridization of calcium/calmodulin dependent protein kinase II and tau mRNAs; species differences and relative preservation in Alzheimer’s disease. Brain Res Mol Brain Res 1992, 12:85-94 [DOI] [PubMed] [Google Scholar]
- 46.Yasojima K, McGeer EG, McGeer PL: Tangled areas of Alzheimer brain have upregulated levels of exon 10 containing tau mRNA. Brain Res 1999, 831:301-305 [DOI] [PubMed] [Google Scholar]
- 47.Chambers CB, Sigurdsson EM, Hejna MJ, Lorens SA, Lee JM, Muma NA: Amyloid-β injection in rat amygdala alters tau protein but not mRNA expression. Exp Neurol 2000, 162:158-170 [DOI] [PubMed] [Google Scholar]
- 48.Murakami N, Oyama F, Gu Y, McLennan IS, Nonaka I, Ihara Y: Accumulation of tau in autophagic vacuoles in chloroquine myopathy. J Neuropathol Exp Neurol 1998, 57:664-673 [DOI] [PubMed] [Google Scholar]
- 49.Oyama F, Murakami N, Ihara Y: Chloroquine myopathy suggests that tau is degraded in lysosomes: implication for the formation of paired helical filaments in Alzheimer’s disease. Neurosci Res 1998, 31:1-8 [DOI] [PubMed] [Google Scholar]
- 50.Cataldo AM, Barnett JL, Pieroni C, Nixon RA: Increased neuronal endocytosis and protease delivery to early endosomes in sporadic Alzheimer’s disease: neuropathological evidence for a mechanism of increased β-amyloidogenesis. J Neurosci 1997, 17:6142-6151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang GP, Khatoon S, Iqbal K, Grundke-Iqbal I: Brain ubiquitin is markedly elevated in Alzheimer disease. Brain Res 1991, 566:146-151 [DOI] [PubMed] [Google Scholar]
- 52.Lopez Salon M, Morelli L, Castano EM, Soto EF, Pasquini JM: Defective ubiquitination of cerebral proteins in Alzheimer’s disease. J Neurosci Res 2000, 62:302-310 [DOI] [PubMed] [Google Scholar]
- 53.Pei J-J, Tanaka T, Tung T-C, Braak E, Iqbal K, Grundke-Iqbal I: Distribution, levels and activity of glycogen synthase kinase-3 in the Alzheimer disease brain. J Neuropathol Exp Neurol 1997, 56:70-78 [DOI] [PubMed] [Google Scholar]
- 54.Pei J-J, Braak E, Braak H, Grundke-Iqbal I, Iqbal K, Winblad B, Cowburn RF: Distribution of active glycogen synthase kinase 3β(GSK-3β) in brains staged for Alzheimer disease neurofibrillary changes. J Neuropathol Exp Neurol 1999, 58:1010-1019 [DOI] [PubMed] [Google Scholar]
- 55.Sironi JJ, Yen SH, Gondal JA, Wu Q, Grundke-Iqbal I, Iqbal K: Ser-262 in human recombinant tau protein is a markedly more favorable site for phosphorylation by CaMKII than PKA or PhK. FEBS Lett 1998, 436:471-475 [DOI] [PubMed] [Google Scholar]
- 56.Bennecib M, Gong C-X, Grundke-Iqbal I, Iqbal K: Inhibition of PP-2A upregulates CaMKII in rat forebrain and induces hyperphosphorylation of tau at Ser 262/356. FEBS Lett 2001, 490:15-22 [DOI] [PubMed] [Google Scholar]
- 57.Nagy Z, Esiri MM, Cato AM, Smith AD: Cell cycle markers in the hippocampus in Alzheimer’s disease. Acta Neuropathol (Berl) 1997, 94:6-15 [DOI] [PubMed] [Google Scholar]
- 58.Pei J-J, Grundke-Iqbal I, Iqbal K, Bogdanovic N, Winblad B, Cowburn RF: Accumulation of cyclin-dependent kinase 5 (cdk5) in neurons with early stages of Alzheimer’s disease neurofibrillary degeneration. Brain Res 1998, 797:267-277 [DOI] [PubMed] [Google Scholar]
- 59.Dranovsky A, Vincent I, Gregori L, Schwarzman A, Colflesh D, Enghild J, Strittmatter W, Davies P, Goldgaber D: Cdc2 phosphorylation of nucleolin demarcates mitotic stages and Alzheimer’s disease pathology. Neurobiol Aging 2001, 22:517-528 [DOI] [PubMed] [Google Scholar]
- 60.Vincent I, Bu B, Hudson K, Husseman J, Nochlin D, Jin L: Constitutive Cdc25B tyrosine phosphatase activity in adult brain neurons with M phase-type alterations in Alzheimer’s disease. Neuroscience 2001, 105:639-650 [DOI] [PubMed] [Google Scholar]
- 61.Wang J, Tung YC, Wang Y, Li XT, Iqbal K, Grundke-Iqbal I: Hyperphosphorylation and accumulation of neurofilament proteins in Alzheimer disease brain and in okadaic acid-treated SY5Y cells. FEBS Lett 2001, 507:81-87 [DOI] [PubMed] [Google Scholar]
- 62.Vincent I, Jicha G, Rosado M, Dicksom DW: Aberrant expression of mitotic cdc2/cyclin B1 kinase in degenerating neurons of Alzheimer’s disease brain. J. Neurosci 1997, 17:3588-3598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Busser J, Geldmacher DS, Herrup K: Ectopic cell cycle proteins predict the sites of neuronal cell death in Alzheimer’s disease brain. J Neurosci 1998, 18:2801-2807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gomez N, Cohen P: Dissection of the protein kinase cascade by which nerve growth factor activates MAP kinases. Nature 1991, 353:170-173 [DOI] [PubMed] [Google Scholar]
- 65.Ferreira A, Lu Q, Orecchio L, Kosik KS: Selective phosphorylation of adult tau isoforms in mature hippocampal neurons exposed to fibrillar Aβ. Mol Cell Neurosci 1997, 9:220-234 [DOI] [PubMed] [Google Scholar]
- 66.Lewis J, Dickson DW, Lin WL, Chisholm L, Corral A, Jones G, Yen SH, Sahara N, Skipper L, Yager D, Eckman C, Hardy J, Hutton M, McGowan E: Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science 2001, 293:1487-1491 [DOI] [PubMed] [Google Scholar]
- 67.Gotz J, Chen F, van Dorpe J, Nitsch RM: Formation of neurofibrillary tangles in P3011 tau transgenic mice induced by Aβ 42 fibrils. Science 2001, 293:1491-1495 [DOI] [PubMed] [Google Scholar]
- 68.Fratiglioni L: Epidemiology of Alzheimer’s disease: issues of etiology and validity. Acta Neurol Scand 1993, 45(Suppl 1):1-70 [PubMed] [Google Scholar]
- 69.Atzori C, Ghetti B, Piva R, Srinivasan AN, Zolo P, Delisle MB, Mirra SS, Migheli A: Activation of the JNK/p38 pathway occurs in diseases characterized by tau protein pathology and is related to tau phosphorylation but not to apoptosis. J Neuropathol Exp Neurol 2001, 60:1190-1197 [DOI] [PubMed] [Google Scholar]
- 70.Pei J-J, Braak E, Braak H, Grundke-Iqbal I, Iqbal K, Winblad B, Cowburn RF: Localization of active forms of C-jun kinase (JNK) and p38 kinase in Alzheimer’s disease brains at different stages of neurofibrillary degeneration. J Alzheimer Dis 2001, 3:41-48 [DOI] [PubMed] [Google Scholar]
- 71.Pei J-J, Braak H, An W-L, Winblad B, Cowburn RF, Iqbal K, Grundke-Iqbal I: Up-regulation of mitogen-activated protein kinases ERK1/2 and MEK1/2 is associated with the progression of neurofibrillary degeneration in Alzheimer’s disease. Mol Brain Res 2002, 109:45-55 [DOI] [PubMed] [Google Scholar]
- 72.Gong CX, Shaikh S, Wang JZ, Zaidi T, Grundke-Iqbal I, Iqbal K: Phosphatase activity toward abnormally phosphorylated tau: decrease in Alzheimer disease brain. J Neurochem 1995, 65:732-738 [DOI] [PubMed] [Google Scholar]
- 73.Gong C-X, Lidsky T, Wegiel J, Zuck L, Grundke-Iqbal I, Iqbal K: Phosphorylation of microtubule-associated protein tau is regulated by protein phosphatase 2A in mammalian brain. Implications for neurofibrillary degeneration in Alzheimer’s disease. J Biol Chem 2000, 275:5535-5544 [DOI] [PubMed] [Google Scholar]
- 74.Bennecib M, Gong CX, Grundke-Iqbal I, Iqbal K: Role of protein phosphatase-2A and -1 in the regulation of GSK-3, cdk5 and cdc2 and the phosphorylation of tau in rat forebrain. FEBS Lett 2000, 485:87-93 [DOI] [PubMed] [Google Scholar]
- 75.Wang J-Z, Gong C-X, Zaidi T, Grundke-Iqbal I, Iqbal K: Dephosphorylation of Alzheimer paired helical filaments by protein phosphatase-2A and -2B. J Biol Chem 1995, 270:4854-4860 [DOI] [PubMed] [Google Scholar]
- 76.Sontag E, Nunbhakdi-Craig V, Bloom GS, Mumby MC: A novel pool of protein phosphatase 2A is associated with microtubules and is regulated during the cell cycle. J Cell Biol 1995, 128:1131-1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Westphal RS, Coffee RL, Jr, Marotta A, Pelech SL, Wadzinski BE: Identification of kinase-phosphatase signaling modules composed of p70 S6 kinase-protein phosphatase 2A (PP2A) and p21-activated kinase-PP2A. J Biol Chem 1999, 274:687-692 [DOI] [PubMed] [Google Scholar]
- 78.Petritsch C, Beug H, Balmain A, Oft M: TGF-β inhibits p70 S6 kinase via protein phosphatase 2A to induce G (1) arrest. Genes Dev 2000, 14:3093-3101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu WK, Moore WT, Williams RT, Hall FL, Yen SH: Application of synthetic phospho- and unphospho-peptides to identify phosphorylation sites in a subregion of the tau molecule, which is modified in Alzheimer’s disease. J Neurosci Res 1993, 34:371-376 [DOI] [PubMed] [Google Scholar]
- 80.Otvos L, Jr., Feiner L, Lang E, Szendrei GI, Goedert M, Lee VM: Monoclonal antibody PHF-1 recognizes tau protein phosphorylated at serine residues 396 and 404. J Neurosci Res 1994, 39:669-673 [DOI] [PubMed] [Google Scholar]
- 81.Su JH, Cummings BJ, Cotman CW: Early phosphorylation of tau in Alzheimer’s disease occurs at Ser-202 and is preferentially located within neurites. Neuroreport 1994, 5:2358-2362 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.