Abstract
Endothelin-1 (ET-1) and its receptors are overexpressed in human Kaposi’s sarcoma lesions. Here we show that in human KS IMM cell line ET-1 increased secretion and activation of matrix-metalloproteinase-2 (MMP-2), -3, -7, -9 and -13, as well as of membrane-type 1-MMP (MT1-MMP). ET-1 and ET-3 also enhanced the expression of tissue inhibitor of MMP-2, essential for MT1-MMP-mediated MMP-2 activation. Combined addition of both ETB receptor (ETBR) and ETAR antagonists completely blocked the ET-1-induced MMP activity. By immunohistochemistry, we observed that ET-1 increased MMP-2 and MT1-MMP expression and their localization at the cell surface. Treatment with both antagonists resulted also in the suppression of ET-1-induced phosphorylation of focal adhesion proteins, FAK and paxillin, which are essentials for cell motility. ET-1 induced a dose-dependent enhancement in KS IMM cell migration and MMP-dependent invasiveness that were inhibited by ET-1 receptor antagonists. The small molecule, A-182086, an orally bioavailable ETA/BR antagonist, completely inhibited cell proliferation and tumor growth in KS IMM xenografts. These findings demonstrate that ET-1-driven autocrine loop is crucial for enhanced invasiveness of KS IMM cells and promote tumor growth in vivo. Such activities can be blocked by the ETA/BR antagonists, which may be effective anti-angiogenic and anti-tumor molecules for the treatment of Kaposi’s sarcoma.
Kaposi’s sarcoma (KS) is an angioproliferative disease associated with KSHV/HHV8 herpesvirus infection. 1 The spindle-shaped cells, the proliferative component of the lesion, are considered to be the tumor cells of endothelial origin. They secrete different chemotactic and angiogenic factors, including vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), interleukins (IL)-6 and IL-8, which are critical for spindle cell proliferation, endothelial cell migration, invasiveness and gelatinase production in vitro, and for the KS lesion development in vivo. 2
We have previously demonstrated that, among other angiogenic factors, endothelin-1 (ET-1) contributes to the pathogenesis of KS. 3 ET-1 is a peptide produced by endothelial and vascular smooth muscle cells and in elevated amounts by tumor cells. 4 ET-1 acts through two distinct subtypes of G-protein-coupled receptors, namely ETA and ETB. The ETA receptor (ETAR) is highly specific for ET-1 whereas ETBR binds both ET-1 and ET-3. 4 Aberrant ET-1-induced cell proliferation and/or survival are implicated in the pathogenesis of many malignancies, including ovarian carcinoma. 5 In this tumor, engagement of ETAR by ET-1 triggers activation of signaling pathways linked to tumor cell proliferation, 6,7 apoptosis protection, 8 invasiveness, 9 and to angiogenesis. 10,11
Previously it has been demonstrated that KS IMM cells, an immortalized KS-derived cell line that retains most of the features of KS and is tumorigenic in nude mice, expresses in vitro and in vivo ETA and ETB receptors, and secrete the peptide ET-1 that acts as an autocrine growth factors. By immunohistochemistry in human KS biopsies we observed a significant expression of ET-1 and its receptors in KS cells and on vessels adjacent to tumor cell nests. These findings together with the inhibitory effect of ET-1 receptor antagonists on cell proliferation, suggest that ET-1 plays an important role in the KS pathogenesis. In addition we demonstrated that ET-1 induces angiogenic responses including proliferation, migration, invasion, gelatinase production and morphogenesis in cultured endothelial cells through ETBR, and that it is capable of stimulating angiogenesis in vivo. 12 These results indicate that ET-1 can promote KS growth by regulating functions of both KS spindle cells and associated endothelial cells.
Increasing numbers of reports have documented the presence of matrix-metalloproteinases (MMPs) in aggressive and invasive tumors 13 and that a high ratio of MMP-2 and tissue inhibitor of MMP-2 (TIMP-2) is associated with poor clinical outcome in different malignancies. 14-17 Previous studies aimed at characterizing MMP expression and activity in KS cell cultures has demonstrated the expression of MMP-1, -2, -3, and -9. 18 In human KS lesions, MMP-2 mRNA is highly expressed and high levels of this enzyme are circulating in KS patients. 19,20 The putative physiological activators of pro-MMP-2 are the membrane-type 1 metalloproteinases (MT1-MMP). 21 In this study we investigated whether ET-1 and ET-3 could induce MMP activation and KS cell migration and invasion. The results indicate that ETs acting through ETAR and ETBR induce the secretion and activity of MMP-2 by increasing the level of its activators, MT1-MMP and TIMP-2. These effects are associated with the induction of KS cell migration and invasiveness, which are blocked by a specific MMP inhibitor. The tyrosine phosphorylation of focal adhesion proteins such as paxillin or focal adhesion kinase (FAK) are essential processes in ET-1-induced signaling pathways. 6,22 Therefore, we analyze the effect of ET-1 receptor antagonists on the phosphorylation of these proteins. In view of these findings, the ET-1 receptors have been proposed as potential targets for anticancer therapy, also in view of the development of non-peptide compounds, capable of blocking ligand-induced activation of the ETAR and/or ETBR. 23-27 Among various ET-1 receptor antagonists, we use ABT-627 (atrasentan), an ETA-selective receptor antagonist, A-192621, an ETB-selective receptor antagonist, and A-182086, an ETA/BR antagonist, to determine their antitumor activity in vitro and in vivo resulting from the blockade of autocrine signal transduction pathways implicated in KS tumor growth and progression.
Materials and Methods
Cells
KS IMM cells were derived from a KS lesion of a transplant patient. 28 KS IMM were grown in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal calf serum (FCS) and 1% penicillin-streptomycin. All culture reagents were from Invitrogen (Paisley, Scotland).
Preparation of Cell-Conditioned Medium
Subconfluent cultures of KS IMM cells were starved for 24 hours in FCS-free DMEM. After the addition of ET-1 or ET-3 100 nmol/L (Peninsula Laboratories, Belmont, CA), the cells were incubated for an additional 24 hours. When the effects of ETAR and/or ETBR antagonist were tested, BQ123 and BQ788 were added 15 minutes before the agonist. In another set of experiments, we treated KS IMM with ABT-627 (atrasentan), an ETAR antagonist, A-192621, an ETBR antagonist, and A-182086, an ETA/BR antagonist (Abbott Laboratories, Abbott Park, IL), and with the addition of ET-1 100 nmol/L. The conditioned medium was then collected, centrifuged, and stored in aliquots at −20°C. The conditioned medium were then processed for zymography and Western blot.
Reverse Transcriptase-Polymerase Chain Reaction
Total RNA was prepared using the TRIzol reagent (Invitrogen) following the manufacturer’s instruction method. The reverse transcriptase-polymerase chain reaction (RT-PCR) was performed using a SUPERSCRIPT One-Step RT-PCR system (Invitrogen) according to the manufacturer’s instructions. Briefly, 1 μg of RNA was reverse-transcribed. The primer sets used for MMP-2 were 5′-TTTGGACTGCCCCAGACAGG-3′ and 5′-GCTGCGGCCAGTATCAGTGC-3′, for MT1-MMP was 5′-CCCTATGCCAACATCGGTGA-3′ and 5′-TCCATCCATGACTTGGTTTAT-3′. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control and the primer sets used were 5′-TGAAGGTCGGTGTCAACGGA-3′, and 5′-GATGGCATGGACTGTGGTCAT-3′. Each RT-PCR included a cDNA synthesis and pre-denaturation cycle at 55°C for 30 minutes and at 94°C for 2 minutes; the cDNA was amplified for 30 cycles of a denaturation step at 94°C for 1 minute; a primer annealing step at 54°C for 30 seconds (MMP-2), at 60°C for 30 seconds (MT1-MMP), at 62°C for 30 seconds (GAPDH); and an extension step at 72°C for 1 minute. The PCR products were analyzed by electrophoresis on a 2% agarose gel containing ethidium bromide and visualized and photographed under UV light.
Northern Blotting
Total RNA from KS IMM cells was extracted using TRIzol (Invitrogen) method according to the manufacturer’s instructions, separated by electrophoresis on 2% denaturing formaldehyde agarose gel (15 μg RNA/lane), and transferred to a nylon membrane. The membranes were UV cross-linked and hybridized in the QuikHyb Hybridization Solution (Stratagene, La Jolla, CA) according to the manufacturer’s instructions. The cDNA probe used for analysis of the MT1-MMP and GAPDH mRNA was prepared using reverse transcription-PCR products. Probes were labeled with [α-32P] dCTP using a random primer oligolabeling kit (Amersham Pharmacia Biotech., Little Chalfont Buckinghamshire, UK) according to the manufacturer’s instructions. Densitometric scanning was performed with a Mustek MFS-6000CX apparatus (Mustek GmbH, Neuss, Germany), and the data were analyzed with Phoretix 1D software and normalized to those of GAPDH.
Western Blotting
Twenty μl of concentrated medium diluted with an equal amount of Laemmli (Bio-Rad Laboratories, Richmond, CA) buffer were electrophoresed on an 11% SDS-polyacrylamide gel. Anti-MMP-2 and anti-MMP-9 antibodies (NeoMarkers, Fremont, CA) were used at a 1:400 dilution. Anti-TIMP-2, anti-MMP-3, anti-MMP-7, anti-MMP-13 antibodies (Chemicon International, Temecula, CA) were used at a 1:1000 dilution. To test the presence of MT1-MMP, 30 μg of whole protein extracts from cell untreated or treated with 100 nmol/L ET-1, 1 μmol/L ABT-627, 1 μmol/L A-192621, and 1 μmol/L A-182086, were separated by 11% SDS-PAGE and revealed by an anti-MT1-MMP antibody (Chemicon) used at a 1:1000 dilution. Peroxidase-labeled secondary antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) was used according to manufacturer’s instructions. Blots were developed with an enhanced chemiluminescence detection system (ECL) kit (Amersham Pharmacia Biotech) according to the manufacturer’s instructions.
Gelatin Zymography
The KS IMM cell supernatants were electrophoresed for analysis in 9% SDS-PAGE gels containing 1 mg/ml gelatin. The gels were then washed for 30 minutes at 22°C in 2.5% Triton X-100 and then incubated in 50 mmol/L Tris (pH 7.6), 1 mmol/L ZnCl2, 5 mmol/L CaCl2 for 18 hours at 37°C. After incubation the gels were stained with 0.2% Coomassie blue. Enzyme-digested regions were identified as white bands on a blue background and quantified by computerized image analysis on the band.
Immunoprecipitation and SDS-PAGE
KS IMM cells were grown to ∼80% confluence and then were serum-starved for 24 hours. After the addition of ET-1 100 nmol/L for a selected period, the cells were rapidly washed with ice-cold PBS and lysed with 0.8 ml of ice-cold lysis buffer [50 mmol/L Tris-HCl (pH 7.4), 100 mmol/L NaCl, 50 mmol/L sodium fluoride, 5 mmol/L EDTA, 1 mmol/L orthovanadate, 0.06 units of aprotinin, 1 mmol/L phenylmethylsulfonyl fluoride, and 10 μg/ml leupeptin]. After centrifugation for 10 minutes at 10,000 rpm, the lysates were preclared for 30 minutes at 4°C by incubation with protein A-Sepharose CL-4B (Pharmacia, Uppsala, Sweden), and immunoprecipitation was performed by incubation for 1.5 hours at 4°C with Abs insolubilized on protein A-Sepharose CL-4B. The immunoprecipitates were washed six times with lysis buffer, solubilized in 2% SDS Laemmli buffer under reducing conditions, and 50 μg/lane were loaded on a 7.5% polyacrylamide gels (SDS-PAGE). The blots were incubated for 1 hour with antiphosphotyrosine monoclonal Ab (0.5 μg/ml; clone 4G10; Upstate Biotechnology Incorporated, Lake Placid, NY). Peroxidase-labeled secondary antibody (Santa Cruz Biotechnology, Inc.) was used according to manufacturer’s instructions. Blots were developed with ECL.
ELISA
MMP-2 in conditioned medium was measured by a Biotrak Human MMP-2 ELISA kit (Pharmacia), following the manufacturer’s instructions. MMP-2 may be measured in the range 1.5 to 24 ng/ml and the sensitivity of the assay is the 0.37 ng/ml.
TIMP-2 in the conditioned media were measured using a human TIMP-2 Immunoassay kit (Chemicon International), following the manufacturer’s instructions. TIMP-2 may be measured in the range 20 to 320 ng/ml and the sensitivity of the assay is 20 ng/ml. The experiments were performed in duplicate and repeated three times.
Immunocytochemistry of MMP-2 and MT1-MMP
KS IMM cells were grown to 80% confluence, harvested, and treated for 24 hours with 100 nmol/L ET-1 or 20 nmol/L phorbol-12-myristate-13-acetate (PHA; Sigma) used as positive control. Indirect immunofluorescence was performed on cytospin preparations according standard procedures using the following primary antibodies: murine monoclonal antibody to MMP-2 (Oncogene Research Products, Boston, MA), polyclonal goat antiserum to MT1-MMP (Santa Cruz Biotechnology), polyclonal rabbit antiserum to TIMP-2 c-terminal (Sigma). The antisera were used at concentrations of 10 μg/ml, 20 μg/ml, and 50 μg/ml, respectively, which were selected on appropriate positive controls. FITC-labeled secondary antisera were obtained from Sigma and used at optimal dilutions with an F/P ratio of 2.5. Slides were observed with a Leitz Orthoplan UV microscope equipped with a ×54 immersion objective. Photographs were taken with Kodak T-Max 400 Pro black and white films.
Chemotaxis and Chemoinvasion Assay
Chemotaxis and chemoinvasion were assessed using a 48 well-modified Boyden chamber (NeuroProbe, Pleasanton, CA) and 8-μm pore PVP-free polycarbonate Nucleopore filters (Costar, New York, NY) as previously described. 29 For chemotaxis, the filters were coated with gelatin by overnight immersion in a solution of 100 mg/ml gelatin in 0.1% acetic acid and then dried. For the chemoinvasion assay, the filters were coated with an even layer of 0.5 mg/ml Matrigel (Becton Dickinson, Bedford, MA). The lower compartment of chamber was filled with chemoattractants or inhibitor (27 μl/well). Serum-starved KS IMM cells (2.5 × 106 cells/ml) were harvested in a trypsin/ethylenediaminetetraacetic (EDTA) acid solution, collected by centrifugation, resuspended in DMEM and placed in the upper compartment (55 μl/well). Where indicated, the cells were preincubated for 15 minutes at 37°C with the ETAR antagonist BQ123 (Peninsula Laboratories) and/or BQ788 (Peninsula Laboratories), an ETBR antagonist. After 4 hours (chemotaxis) or 6 hours (chemoinvasion) of incubation at 37°C, the filters were removed, stained with Diff-Quick (Merz-Dade, Dudingen, Switzerland), and the migrated cells in 10 high-power fields were counted. Each experimental point was analyzed in triplicate. In selected experiments, invasion was quantified in the presence of Ilomastat (GM6001, Chemicon International), a broad-spectrum chemical inhibitor of MMP activity.
Cell Proliferation Assay
KS IMM cells were seeded in 96-well plates at 80% confluence (1 × 104 cells/well) and incubated in serum-free DMEM for 24 hours to induce quiescence. Then, cells were treated with ABT-627 (1 μmol/L), A-192621 (1 μmol/L), or A-182086 (1 μmol/L) in the absence or presence of 100 nmol/L ET-1. After 24 hours 1 μCi of [methyl]-[3H] thymidine (6.7 Ci/mmol; Dupont, New England Nuclear Research Products, Wilmington, DE) was added to each well. ET receptor antagonists were incubated 15 minutes before the addition of ET-1. Six hours later the culture media were removed and the cells were washed three times with PBS, treated with 10% trichloroacetic acid for 15 minutes, washed twice with 100% ethanol, and solubilized in 0.4 N sodium hydroxide. The cell-associated radioactivity was then determined by liquid scintillation counting. Responses to all agents were assayed in sextuplicate and results were expressed as means of three separate experiments.
KS IMM Xenografts in Nude Mice
Female athymic (nu+/nu+) mice, 4 to 6 weeks of age, were purchased from Charles River Laboratories (Milan, Italy). The treatment protocol followed the guidelines of animal experimentation adopted by the Regina Elena Cancer Institute under the control of the Ministry of Public Health. Mice were injected s.c. on one flank with 3 × 106 viable KS IMM cells, as determined by trypan blue staining, resuspended in 200 μl of PBS. The mice were randomized in groups (n = 10) to receive different treatments. Animals were treated by i.p. injection with ABT-627, with A-19262, and with A-182086. The treatment was started 7 days after the xenograft, ending on day 21, and each experiment was repeated three times. In each experiment, one group was treated i.p. for 21 days with ABT-627 (2 mg/kg/day), one group was treated i.p. for 21 days with A-192621 (10 mg/kg/day), and one group was treated i.p. with A-182086 (2 mg/kg/day). Control mice were injected in the same way with 200 μl of drug vehicle. Tumor size was measured with calipers and was calculated using the formula π/6 × larger diameter × (smaller diameter)2.
Statistical Analysis
Statistical evaluations of data were made by the two-sided Student’s test and analysis of variance (ANOVA) as appropriate.
Results
ET-1 Induces Secretion and Activation of MMP-2 in KS IMM Cells
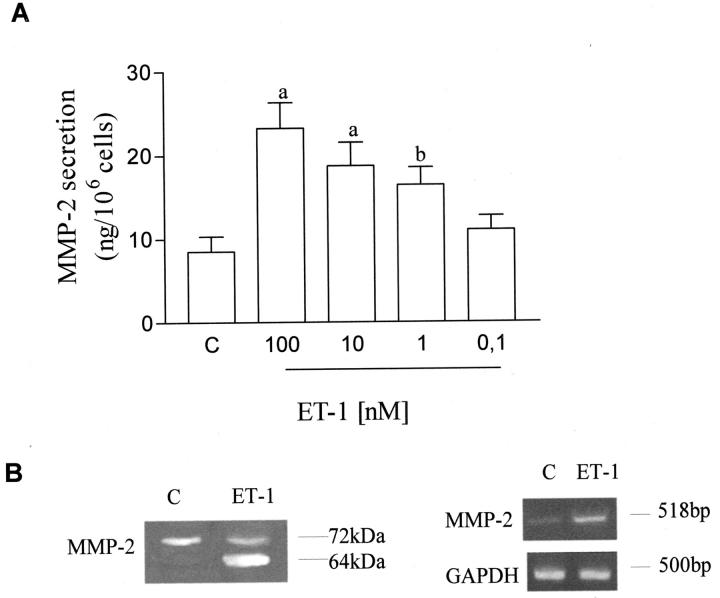
MMP-2 is highly expressed by KS and is released as a latent proenzyme (72 kDa) which is proteolytically cleaved to an active 64-kDa form through a complex mechanism involving other proteases. 21 Using a human MMP ELISA kit, we measured the effect of different concentrations of ET-1 on the secretion of MMP-2 by KS IMM cell line. As shown in Figure 1A ▶ , ET-1 enhanced MMP-2 secretion in a dose-dependent manner in the range between 0.1 nmol/L to 100 nmol/L. To assess the effect of ET-1 on KS IMM gelatinase A (MMP-2) secretion and activation in vitro, the MMP-2 protein released by KS IMM cells untreated or treated with 100 nmol/L of ET-1 for 24 hours were analyzed by gelatin zymography. Unstimulated cells expressed the characteristic gelatinase activities corresponding to the 72,000 kDa (pro-MMP-2), whereas activated forms of this enzyme were not detectable in control samples (Figure 2B) ▶ . ET-1-treated cells showed a striking increase of the bands corresponding to the molecular weight of the active (64 kDa) form of MMP-2.
Figure 1.
Effects of ET-1 on the synthesis, secretion and activation of MMP-2 in KS IMM cells. A: ET-1 stimulates MMP-2 secretion from KS IMM cells. MMP-2 secretion was measured in conditioned media from cells treated with different concentrations of ET-1 for 24 hours using ELISA kit. Data are presented as means of results from three experiments each performed in duplicate. Bars ± SD. a, P ≤ 0.0001; b, P ≤ 0.005. B: Left, gelatinolytic activity of MMP-2 was studied in conditioned media from cells by SDS-PAGE gelatin zymography. Cells, after starvation, were grown in serum-free medium for 24 hours in the absence (C; control) or presence of 100 nmol/L ET-1. Right, expression of 518 mRNA transcripts for MMP-2 was detected by RT-PCR analysis. Primers for the amplification of GAPDH gene were used as controls. Data shown are PCR products of KS IMM cells grown in serum-free medium for 8 hours in the absence (C; control) and in presence of 100 nmol/L of ET-1. PCR products for MMP-2 and GAPDH were shown as visualized by ethidium bromide.
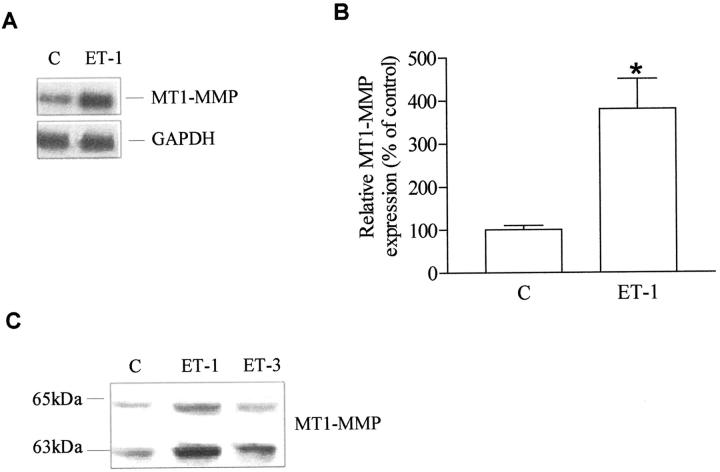
Figure 2.
Effect of ET-1 on the synthesis, secretion, and activation of MT1-MMP in KS IMM cells. A: Total RNA (20 μg) extracted from untreated cells (C; control) or treated for 8 hours with 100 nmol/L ET-1 was analyzed by Northern blotting using MT1-MMP cDNA probe. The filter was also hybridized with a GAPDH probe, as a control for RNA loading. B: Fold-increase of MT1-MMP mRNA over control by densitometric analysis of autoradiographic bands normalized to those of GAPDH. Bars ± SD; *, P ≤ 0.001 compared to control. C: Extracts of untreated KS IMM cells (C; control) or treated with 100 nmol/L ET-1 or ET-3 for 24 hours was tested for MT1-MMP (the 65-kd latent form and the 63-kd active form) by Western blotting.
At the transcriptional level, ET-1 (100 nmol/L) up-regulated MMP-2 mRNA expression in KS IMM cells (Figure 1B ▶ , right), as determined by densitometric analysis and comparison with the intensity of the PCR products corresponding to GAPDH.
ET-1 Induces MT1-MMP Secretion and Activation in KS IMM Cells
MT1-MMP is a transmembrane MMP known to bind and activate MMP-2 at the cell surface. To investigate the effect of ET-1 on MT1-MMP mRNA transcripts, Northern blot analysis was performed on KS IMM cells untreated or treated with 100 nmol/L ET-1 (Figure 2A) ▶ . Densitometric analysis of the bands revealed a slight up-regulation (3.8-fold) of the transcripts for MT1-MMP in ET-1-treated cells (Figure 2B) ▶ . ET-1 enhanced expression of both the latent MT1-MMP (65 kDa) and, to an even greater extent, the activated form of MT1-MMP (63 kDa) as compared with untreated KS IMM cells (Figure 2C) ▶ , as determined by Western blot. Addition of ET-3, a ligand for ETBR with the same affinity as ET-1, also increased the level of activated MT1-MMP, indicating that both ET-1 and ET-3 induce the MT1-MMP activation required to mediate cell surface pro-MMP-2 activation.
ETA and ETB Receptor Antagonists Block MMPs and MT1-MMP Activation in KS IMM Cells
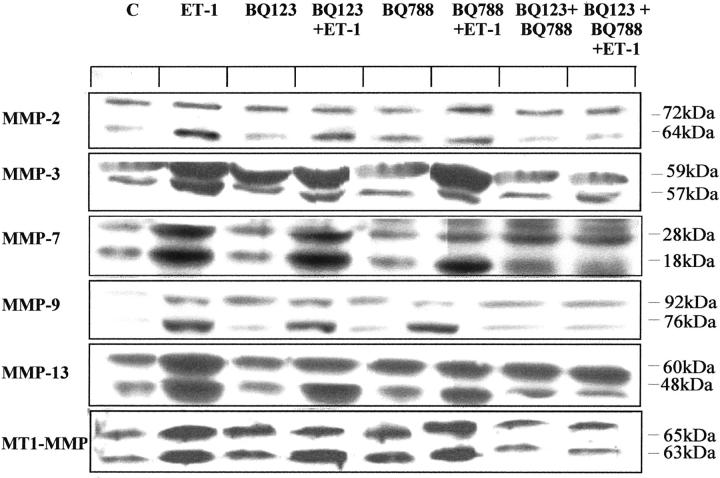
The effect of ET-1 on the secretion and activation of other metalloproteinases (MMP-3, MMP-7, MMP-9, and MMP-13) by KS IMM cells was analyzed by Western blotting. Conditioned media of untreated cells exhibited both latent and active forms of MMP-3 (59-kDa and 57-kDa forms), MMP-7 (28-kDa and 18-kDa forms), MMP-9 (92-kDa and 76-kDa forms) and MMP-13 (60-kDa and 48-kDa forms) (Figure 3) ▶ . Treatment with 100 nmol/L ET-1 induced over-expression of both latent and active forms of MMP-3, -7, -9, and -13 in KS IMM cells as compared to untreated cells.
Figure 3.
ET-1-induced MMP expression is mediated by both ETAR and ETBR. Conditioned media or cell lysates from untreated cells (C; control) or with addition of 100 nmol/L ET-1 were tested for MMP-2 (the 72-kDa proform and 64-kDa active form), MMP-3 (the 59-kDa proform and the 57-kDa active form), MMP-7 (the 28-kDa proform and the 18-kDa active form), MMP-9 (the 92-kDa proform and the 76-kDa active form), the MMP-13 (the 60-kDa proform and the 48-kDa active form), and the MT1-MMP (the 65-kDa proform and the 63-kDa active form) by Western blotting. KS IMM cells after starvation were pretreated (15 minutes) with ETAR antagonist, BQ 123 (1 μM) or ETBR antagonist, BQ 788 (1 μM), or with a combination of both antagonists in the absence or presence of 100 nmol/L ET-1 for 24 hours.
To characterize the receptor subtype involved in MMP activation, we analyzed the effect of BQ 123, a selective ETAR antagonist, and BQ 788, a selective ETBR antagonist, on MMP activity by Western blotting. Addition of 1 μM BQ 123 or 1 μM BQ 788 only partially inhibited the ET-1-induced release and activation of all MMPs. Interestingly, the addition of both BQ 123 and BQ 788 completely blocked the conversion of latent MMPs to their active form on ET-1 treatment (Figure 3) ▶ . These data indicate that the MMP activation observed was mediated by both the ETA R and ETBR subtypes.
Effects of ET-1 and ET-3 on TIMP-2 Expression in KS IMM Cells
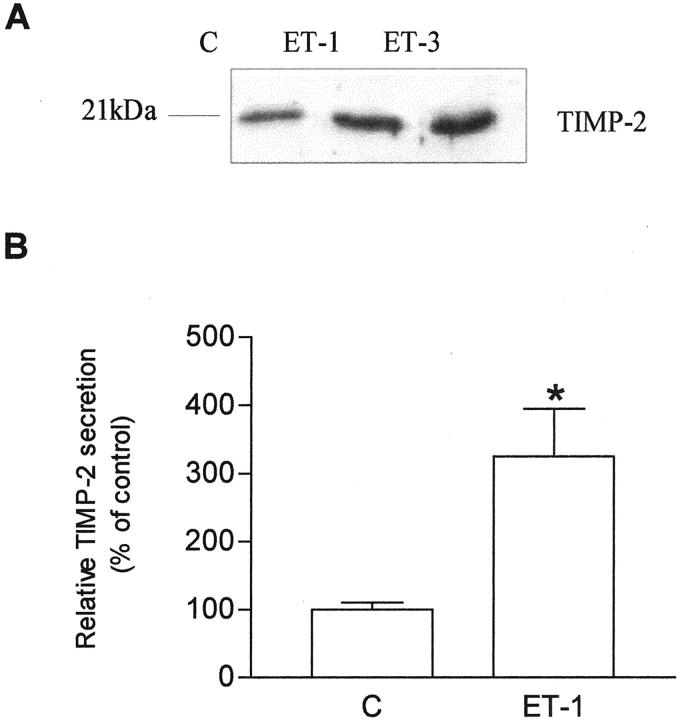
Several studies have demonstrated that activation of pro-MMP-2 by MT1-MMP depends on the presence of critical amounts of TIMP-2, which is required for the formation of the ternary complex that leads to the activation of MMP-2. 30 Both ET-1 and ET-3 induced a significant increase of TIMP-2 expression when compared to untreated cells (Figure 4A) ▶ , as demonstrated by Western blotting. The ET-1-induced stimulation of TIMP-2 expression by KS IMM cells was also measured using an ELISA assay capable of recognizing TIMP-2 complexed with active MMP-2. We observed that TIMP-2 levels were increased by threefold with 100 nmol/L ET-1 compared with untreated control cells (Figure 4B) ▶ . The activation of pro-MMP-2 by MT1-MMP is accelerated in the presence of appropriate amounts of TIMP-2, thus the concomitant association of overexpression of MT1-MMP and TIMP-2 are consistent with the enhanced pro-MMP-2 activation.
Figure 4.
Effects of ET-1 and ET-3 on the secretion of TIMP-2 by KS IMM cells. A: Conditioned media from untreated cells (C; control) or with 100 nmol/L ET-1 or with 100 nmol/L ET-3 were tested for TIMP-2 (21 kDa) by Western blotting. B: TIMP-2 secretion was measured in conditioned media from cells treated with 100 nmol/L ET-1 for 24 hours using ELISA kit. Data are presented as means of results from three experiments each performed in duplicate. Bars ± SD; *, P ≤ 0.001 compared to control.
ET-1 Enhances MMP-2 and MT1-MMP Expression in KS IMM Cells
To define the regulatory activity of ET-1 on MT1-MMP, TIMP-2 and MMP-2, we evaluated the degree and patterns of expression of MMP-2 and MT1-MMP expression in ET-1-treated cells by immunohistochemistry (Figure 5) ▶ . The degree of expression of MMP-2 in control cells (panel A) was highly heterogeneous in intensity and distribution. The fluorescence was clustered in discrete areas of the cytoplasm (arrows) with a fine granular distribution. Following ET-1-treatment, the intensity of staining increased substantially, it appeared more homogenous in the cell population, and showed a coarse granular pattern (panel B). MT1-MMP staining also underwent significant changes after ET-1 exposure (panel D) when compared to control cells (panel C) in terms of increased expression and coarser granular pattern clustered at the periphery of the cells (arrows) suggesting an ET-1-induced change in MT1-MMP/MMP-2 trafficking or localization (Figure 5) ▶ . Increased expression of TIMP-2 was also observed in KS cells on incubation with ET-1 (data not shown). KS cells treated with PMA were used as positive control.
Figure 5.
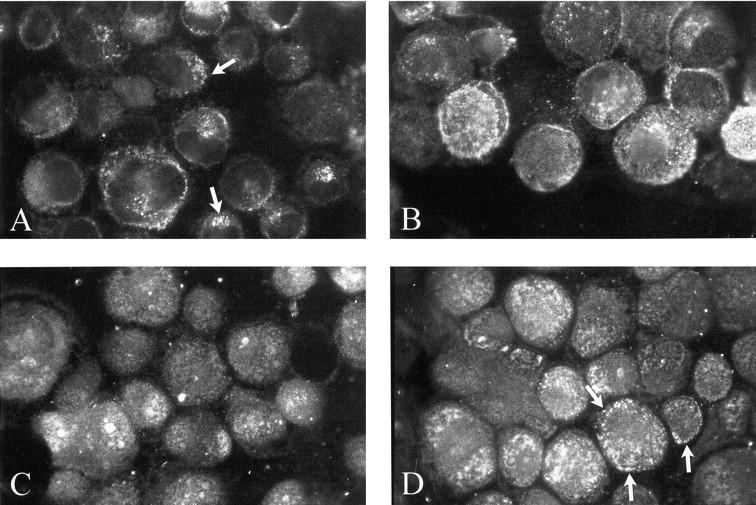
Modulation of MMP-2 and MT1-MMP expression in KS IMM cells following incubation with ET-1. Both MMP-2 (A and B) and MT1-MMP (C and D) undergo an increase in levels of expression and changes in distribution patterns in the presence of 100 nmol/L ET-1 (B and D, respectively) for 24 hours compared to control (A and C, respectively). Original magnification, ×400; FITC.
ET-1 Receptor Blockade Suppresses the ET-1-Induced Tyrosine Phosphorylation of FAK and Paxillin in KS IMM Cells
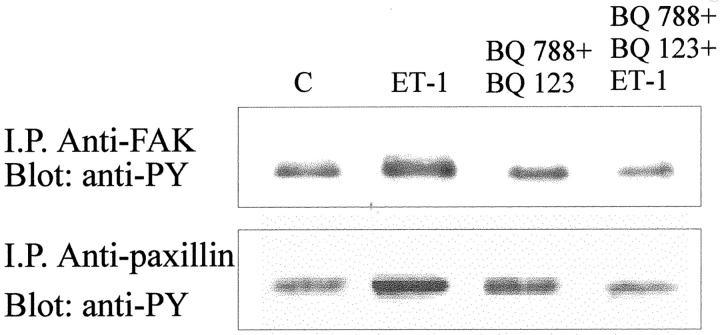
FAK tyrosine phosphorylation occurs during formation of focal adhesions and spreading on extracellular matrix (ECM) 31 and this signaling pathway is activated also by ET-1. 6,22 To determine whether ET-1 is able to increase the tyrosine phosphorylation of 125FAK in KS IMM cells, we immunoprecipitated KS IMM cell lysate with anti-FAK antibody, followed by immunoblotting with anti-phosphotyrosine antibody. We detected a significant and specific increase of tyrosine phosphorylated FAK in KS IMM cells treated with ET-1 100 nmol/L for 5 minutes (Figure 6) ▶ . Because the major targets downstream the FAK phosphorylation is the paxillin, we also investigated the effect of ET-1 on the phosphorylation of this protein linking extracellular matrix to cytoskeleton. Immunoprecipitation with monoclonal antibody to paxillin demonstrated that ET-1 induced also the paxillin tyrosine phosphorylation. The addition of both BQ 123 (1 μmol/L) and BQ 788 (1 μmol/L) suppresses the tyrosine phosphorylation of both focal adhesion proteins (Figure 6) ▶ .
Figure 6.
ET-1 induces tyrosine phosphorylation of FAK and paxillin in KS IMM cells. Cells were serum starved for 24 hours (C), preincubated with BQ 123 and BQ 788 (1 μmol/L), and then stimulated with 100 nmol/L of ET-1 for 5 minutes. Cell lysates were immunoprecipitated with antibodies to FAK and to paxillin and immunoblotted with anti-phosphotyrosine antibody.
Effect of ET-1 on KS IMM Migration and Invasion
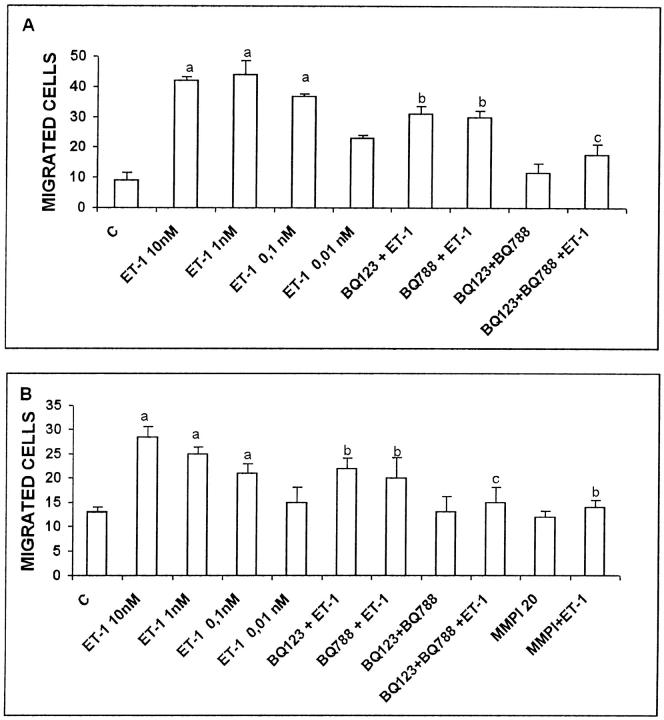
To assess whether ET-1 had the functional effect on the migratory and invasive activities of KS IMM cells, we performed Boyden chamber chemotaxis and invasion assays. Addition of 0.01 to 10 nmol/L ET-1 to the KS IMM cells induced a marked and dose-dependent increase in cell migration (Figure 7A) ▶ . The stimulatory effect of 10 nmol/L ET-1 on KS IMM cell migration was partially inhibited in the presence of 1 μmol/L BQ 123 or BQ 788; exposure of the cells to both BQ 788 and BQ 123 completely blocked the ability of KS IMM cells to respond to ET-1 (Figure 7A) ▶ . In Matrigel-coated invasion chambers, we observed that ET-1 dose-dependently stimulated the invasive activity of KS IMM cells (Figure 7B) ▶ . Again, exposure of the cells to BQ 788 in combination with BQ 123 completely abolished the ability of KS IMM cells to respond to ET-1 (10 nmol/L). To assess if the increased invasion of ET-1-treated KS IMM cells was a functional consequence of enhanced matrix proteolytic potential, in the same experiments we used also the potent chemical broad-spectrum MMP inhibitor Ilomastat. ET-1-stimulated invasion was reduced to the control levels in the presence of 20 μmol/L Ilomastat. Taken together, these results demonstrate that ET-1 is able to induce tumor cell migration and MMP-dependent invasion through both the ETAR and ETBR.
Figure 7.
Effect of ET-1 on KS IMM cell migration and invasion. Columns indicated by a C are controls. KS IMM cells (2.5 × 106 cells/ml) were treated with different doses of ET-1. Cells pretreated with 1 μmol/L of the ETAR antagonist (BQ 123) and/or ETBR antagonist (BQ 788) or with MMP inhibitor (MMPI, 20 μmol/L) were placed in the upper compartment in Boyden chamber in the absence or in the presence of ET-1 (10 nmol/L). Cells migrated through the filter were counted after 4 hours (chemotaxis; A) or cells migrating through the Matrigel layer were counted after 6 hours (chemoinvasion; B). Data are expressed as the number of migrated cells in 10 high-power fields and are means of results from three experiments each performed in triplicate. a, P ≤ 0.001 compared to control. b, P ≤ 0.02 compared to ET-1 10 nmol/L. c, P ≤ 0.001 compared to BQ 123+ET-1 and BQ 788+ET-1.
Effects ET Receptor Blockade on KS IMM Cell Proliferation and Tumor Growth in Vivo
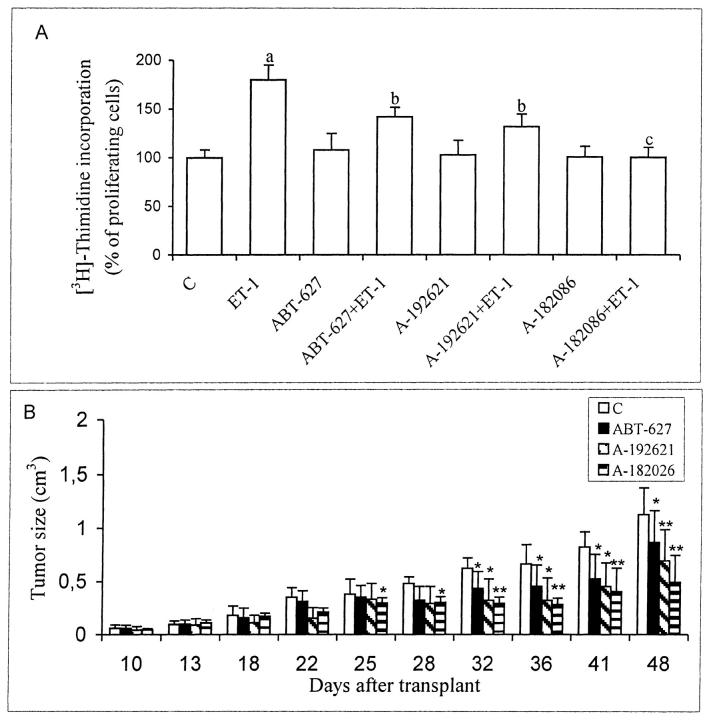
To evaluate the effect of the potent non-peptide ET-1 receptor antagonists on the proliferation of KS IMM cell, we co-incubated the cell with ET-1 and/or with different ETAR and ETBR antagonists. We ascertained that the strong ET-1-induced proliferation (P ≤ 0.001) was partially inhibited in the presence of ABT-627 (1 μmol/L) (P ≤ 0.01), the ETAR antagonist or A-192621 (1 μmol/L) (P ≤ 0.01), the ETBR antagonist, whereas the stimulatory effect is completely inhibited in the presence of A-182086 (1 μmol/L) (P ≤ 0.001), the ETA/BR antagonist (Figure 8A) ▶ . Taken together, these data confirm that the mitogenic signaling by ET-1 is mediated by both receptors and that these effects can be abolished by the orally biovailable ETA/BR antagonist. We translate the results obtained in vitro into a model of nude mice xenografted with KS IMM cells. In all nude mice that were subcutaneously inoculated with 3 × 106 KS IMM cells, a lesion developed at the site of inoculation within 5 to 7 days. Fifteen days following the injection, specimens were taken from the lesional sites. Histologically, the neoplastic lesion consisted of round and spindle cells, with vascular structures and capillaries and some infiltrated inflammatory cells. A strong cytoplasmic staining for ET-1, ETAR, and ETBR was observed in the cultured KS IMM cells and in the KS IMM-derived lesions in nude mice, demonstrating that in vivo KS-derived cells co-expressed ETAR and ETBR that could be specifically targeted by ET-1 receptor antagonists. 3 The animals were treated 7 days after tumor implant, with vehicle or with ABT-627 (2 mg/kg/day), A-192621 (10 mg/kg/day), or A-182086 (2 mg/kg/day) i.p. on days 1 to 21 (Figure 8B) ▶ . Comparison of tumor growth curves by analysis of variance showed that the differences between ABT-627-treated groups, the A192621-treated groups and the control were statistically significant for all measurements made after day 31 (P ≤ 0.02), with a partially suppression of tumor growth. More profound tumor growth inhibition (56% of controls) was elicited in A-182086-treated mice with respect to the control and the differences were statistically significant for all measurements after day 25 (P ≤ 0.02 up to day 32 and then P ≤ 0.001 for all other measurements). These data suggest that also in vivo, the tumor-promoting activity of ET-1 is mediated by both receptor subtypes that can be blocked by A-182086.
Figure 8.
Effects of ET receptor antagonists on ET-1-induced proliferation in vitro and tumor growth in vivo. A: 100 nmol/L of ET-1 was added to quiescent KS IMM cells. ABT-627, the ETAR antagonist (1 μmol/L), A-192621, the ETBR antagonist (1 μmol/L) and A-182086, the ETA/BR antagonist (1 μmol/L), were incubated 15 minutes before the addition of ET-1. [3H]thymidine incorporation was analyzed 24 hours after the addition of the agonist. Data are means of results from three experiments each performed in sextuplicate. a, P ≤ 0.001 compared to control. b, P ≤ 0.01 compared to ET-1 100 nmol/L. c, P ≤ 0.001 compared to ET-1 100 nmol/L. B: Antitumor activity of ET receptor antagonist treatment on established KS IMM human xenografts. Mice were injected s.c. with 3 × 106 KS IMM cells. After 7 days the mice were treated i.p. for 21 days with vehicle, with ABT-627 (2 mg/kg/day), with A-192621 (10 mg/kg/day), or with A-182086 (2 mg/kg/day) i.p. on days 1 to 21, 7 days after tumor implant. Three different experiments with a total of 40 mice for each experiment were performed. In each experiment, each group consisted of 10 mice. Data represent the averages; bars ± SD *, P ≤ 0.02; **, P ≤ 0.001 compared with control.
Discussion
ECM remodeling is one of the most important events in the pathogenesis of tumors, because cancer cells feed themselves by recruiting blood vessels into tumor mass and escape from their original sites by penetrating the basal lamina and other ECM structures. Among the tumor proteases, MMP-2 is highly expressed in KS lesions. 32 We have reported that KS-derived spindle-shaped cells contain and secrete large amounts of ET-1, a multifunctional peptide endowed with growth-stimulatory properties, 5 and express the cognate receptors. In these cells, ET-1 acts as an autocrine growth factor and blocking ET-1/ET-1 receptor interaction leads to inhibition of the mitogenic effect. 3 Here we demonstrated that ET-1 induces MMP-2 synthesis, secretion, and activation. ET-1-treatment in KS IMM cells results in up-regulation of MT1-MMP and MMP-2 not only by inducing a de novo synthesis of latent enzymes, but also by increasing their activation. The enzyme cascade is confined to the cell surface at the point of invading pseudopodia. MT1-MMP contains a transmembrane/cytoplasmic sequence that confines it to micro-invasion sites on the surface of the tumor-cell invadopodia. 33-36 MT1-MMP and MMP-2 immunoreactivity following ET-1 treatment is not only increased but also redistributed with polarization on KS IMM cells. Therefore, it is interesting to speculate that ET-1 may function in part to focalize MT1-MMP proteolityc activity to sites of cell-matrix contact, thus promoting pericellular proteolysis and subsequent invasion. Moreover, ET-1 significantly enhanced the secretion of TIMP-2 which is required for the formation of a ternary complex with pro-MMP-2 and MT1-MMP on the cell surface, allowing MMP-2 activation. We further demonstrated that ET-1 enhances the secretion and activation of other MMPs, such as MMP-3, -7, -9, and -13, leading to the degradation of all known ECM components, thus enabling KS IMM cells to invade the ECM.
The angiogenic process shows a dependence on MMP-2 activity that occurs as an early event to degrade the vascular basement membranes allowing endothelial cell migration and invasion. In this context, we have demonstrated that ET-1 induces a proangiogenic phenotype in human endothelial cells, expressing predominantly ETBR, by stimulating MMP-2 activation, and consequently selective ET-1 receptor antagonists could inhibit ET-1-mediated neovascularization. 22 In this study, we demonstrated that the functional consequence of increased synthesis, secretion, and activation of MMP-2 by ET-1 and ET-3 is the enhanced KS cellular motility and invasiveness, which are abolished by a combination of both ET-1 receptor antagonists, identifying ETs and their receptors as regulators of the activation of MMP-2 in endothelial 10 and in KS cells.
The overexpression and activation of FAK is associated with invasive and metastatic phenotype in many tumors. 31 The current study aimed at elucidating the mechanisms of ETs action on KS IMM cells reveals that ET-1 receptors antagonists inhibited the ET-1-mediated of FAK and paxillin tyrosine phosphorylation. These effects on FAK phosphorylation directly correlate with KS IMM cell migration and invasion, suggesting that blockade of ET-1 receptors resulted in inhibition of cell motility and other FAK-associated processes. Treatment of KS cells with a selective ETA/BR antagonist resulted in suppression of tumor growth in vitro and in vivo. We previously demonstrated that ET-1 and its receptors are expressed by tumor cells as well as by tumor vessels in KS lesions. 3 The tumor-promoting activity of ET-1 and ET-3 may occur through an autocrine pathway that stimulates tumor cell proliferation and through a paracrine pathway involving direct angiogenic effects on endothelial cells. Taken together these data indicate that treatment with ETA/BR antagonist has an antitumor effect in vivo that is attributable to the direct block of pathway-transducing signals involved in proliferation and invasiveness and, in part, to inhibition of angiogenic effect. The ETs and their receptors, referred to as the endothelin axis, represent a new and mostly unexplored target for cancer therapy. 27 Preliminary results from clinical trials, such as those with ABT 627 in prostate cancer showing the oral feasibility and suitable pharmacokinetic and toxicity profiles for clinical use, are encouraging. 37 In this context, new therapeutic strategies using small molecules such as specific ET-1 receptor antagonists provide an additional approach to the treatment of KS in which ET-1 receptor blockade could result in tumor growth inhibition by reducing angiogenesis, tumor cell proliferation, and invasion.
Acknowledgments
We thank Dr. Perry Nisen of Abbott Laboratories (Global Oncology Development, Abbott Park, IL) for kindly providing the ABT-627, A-192621, and A-182086, Marco Varmi and Rolando Rossi for technical assistance and Maria Vincenza Sarcone for excellent secretarial assistance.
Footnotes
Address reprint requests to Anna Bagnato, Laboratory of Molecular Pathology and Ultrastructure, Regina Elena Cancer Institute, Via delle Messi D’Oro 156, 00158 Rome, Italy. E-mail: bagnato@ifo.it.
Supported by grants from the Associazione Italiana Ricerca sul Cancro, from the Italian Ministry of Health and from the CNR-Italian Ministry of Education, University and Research (MIUR). Dr. Francesca Spinella is a recipient of a fellowship from Fondazione Italiana Ricerca sul Cancro.
References
- 1.Antman K, Chang Y: Kaposi’s sarcoma. N Engl J Med 2000, 342:1027-1038 [DOI] [PubMed] [Google Scholar]
- 2.Masood R, Cai J, Tulpule A, Zheng T, Hamilton A, Sharma S, Espina BM, Smith Dl, Gill PS: IL-8 is an autocrine growth factor and a surrogate marker for Kaposi’s sarcoma. Clin Cancer Res 2001, 7:2693-2702 [PubMed] [Google Scholar]
- 3.Bagnato A, Rosanò L, Di Castro V, Albini A, Salani D, Varmi M, Nicotra MR, Natali PG: Endothelin receptor blockade inhibits proliferation of Kaposi’s sarcoma cells. Am J Pathol 2001, 158:841-847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rubanji GM, Polokoff MA: Endothelins: molecular biology, biochemistry, pharmacology, physiology, and pathophysiology. Pharmacol Rev 1999, 46:325-414 [PubMed] [Google Scholar]
- 5.Bagnato A, Catt J: Endothelins as autocrine regulators of tumor cell growth. Trends Endocrinol Metab 1998, 9:378-383 [DOI] [PubMed] [Google Scholar]
- 6.Bagnato A, Tecce R, Di Castro V, Catt KJ: Activation of mitogenic signaling by endothelin 1 in ovarian carcinoma cells. Cancer Res 1997, 57:1306-1311 [PubMed] [Google Scholar]
- 7.Bagnato A, Salani D, Di Castro V, Wu-Wong JR, Tecce R, Nicotra MR, Venuti A, Natali PG: Expression of endothelin-1 and endothelin A receptor in ovarian carcinoma: evidence for an autocrine role in tumor growth. Cancer Res 1999, 59:1-8 [PubMed] [Google Scholar]
- 8.Del Bufalo D, Di Castro V, Biroccio A, Varmi M, Salani D, Rosanò L, Trisciuoglio D, Spinella F, Bagnato A: Endothelin-1 protects ovarian carcinoma cells against paclitaxel-induced apoptosis: requirement for AKT activation. Mol Pharmacol 2002, 61:524-532 [DOI] [PubMed] [Google Scholar]
- 9.Rosanò L, Varmi M, Salani D, Di Castro V, Spinella F, Natali PG, Bagnato A: Endothelin-1 induces tumor proteinase activation and invasiveness of ovarian carcinoma cells. Cancer Res 2001, 61:8340-8346 [PubMed] [Google Scholar]
- 10.Salani D, Taraboletti G, Rosanò L, Di Castro V, Borsotti P, Giavazzi R, Bagnato A: Endothelin-1 induces an angiogenic phenotype in cultured endothelial cells and stimulates neovascularization in vivo. Am J Pathol 2000, 157:1526-1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spinella F, Rosanò L, Di Castro V, Natali PG, Bagnato A: Endothelin-1 induces vascular endothelial growth factor by increasing hypoxia-inducible factor-1α in ovarian carcinoma cells. J Biol Chem 2002, 277:27850-27855 [DOI] [PubMed] [Google Scholar]
- 12.Salani D, Di Castro V, Nicotra MR, Rosanò L, Tecce R, Venuti A, Natali PG, Bagnato A: Role of endothelin-1 in neovascularization of ovarian carcinoma. Am J Pathol 2000, 157:1537-1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noel A, Gilles C, Bajou K, Devy L, Kebers F, Lewalle JM, Maquoi E, Munaut C, Remacle A, Foidart JM: Emerging roles for proteinases in cancer. Invasion Metastasis 1997, 17:221-239 [PubMed] [Google Scholar]
- 14.Jiang Y, Goldberg ID, Shi YE: Complex roles of tissue inhibitors of metalloproteinases in cancer. Oncogene 2002, 21:2245-2252 [DOI] [PubMed] [Google Scholar]
- 15.Yoshizaki T, Maruyama Y, Sato H, Furukawa M: Expression of tissue inhibitor of matrix metalloproteinase-2 correlates with activation of matrix metalloproteinase-2 and predicts poor prognosis in tongue squamous cell carcinoma. Int J Cancer 2001, 95:44-50 [DOI] [PubMed] [Google Scholar]
- 16.Remacle A, McCarthy K, Noel A, Maguire T, McDermott E, O’Higgins N, Foidart JM, Duffy MJ: High levels of TIMP-2 correlate with adverse prognosis in breast cancer. Int J Cancer 2000, 89:118-121 [DOI] [PubMed] [Google Scholar]
- 17.Kallakury BV, Karikehalli S, Haholu A, Sheehan CE, Azumi N, Ross JS: Increased expression of matrix metalloproteinases 2 and 9 and tissue inhibitors of metalloproteinases 1 and 2 correlate with poor prognostic variables in renal cell carcinoma. Clin Cancer Res 2001, 7:3113-3119 [PubMed] [Google Scholar]
- 18.Meade-Tollin LC, Way D, Witte MH: Expression of multiple matrix metalloproteinases and urokinase type plasminogen activator in cultured Kaposi sarcoma cells. Acta Histochem 1999, 101:305-316 [DOI] [PubMed] [Google Scholar]
- 19.Toschi E, Barillari G, Sgadari C, Bacigalupo I, Cereseto A, Carlei D, Palladino C, Zietz C, Leone P, Sturzl M, Butto S, Cafaro A, Monini P, Ensoli B: Activation of matrix-metalloproteinase-2 and membrane-type-1-matrix-metalloproteinase in endothelial cells and induction of vascular permeability in vivo by human immunodeficiency virus-1 Tat protein and basic fibroblast growth factor. Mol Biol Cell 2001, 12:2934-2946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sgadari C, Barillari G, Toschi E, Carlei D, Bacigalupo I, Baccarini S, Palladino C, Leone P, Bugarini R, Malavasi L, Cafaro A, Falchi M, Valdembri D, Rezza G, Bussolino F, Monini P, Ensoli B: HIV protease inhibitors are potent anti-angiogenic molecules and promote regression of Kaposi sarcoma. Nat Med 2002, 8:225-232 [DOI] [PubMed] [Google Scholar]
- 21.Murphy G, Knauper V, Cowell S, Hembry R, Stanton H, Butler G, Freije J, Pendas AM, Lopez-Otin C: Evaluation of some newer matrix metalloproteinases. Ann NY Acad Sci 1999, 878:25-39 [DOI] [PubMed] [Google Scholar]
- 22.Vacca F, Bagnato A, Catt KJ, Tecce R: Transactivation of the epidermal growth factor receptor in endothelin-1-induced mitogenic signaling in human ovarian carcinoma cells. Cancer Res 2000, 60:5310-5317 [PubMed] [Google Scholar]
- 23.Bagnato A, Cirilli A, Salani D, Simeone P, Muller A, Nicotra MR, Natali PG, Venuti A: Growth inhibition of cervix carcinoma cells in vivo by endothelin A receptor blockade. Cancer Res 2002, 62:6381-6384 [PubMed] [Google Scholar]
- 24.Van der Boon J: New drug slows prostate cancer progression. Lancet Oncol 2002, 3:201. [DOI] [PubMed] [Google Scholar]
- 25.Carducci MA, Nelson JB, Bowling MK, Rogers T, Eisenberger MA, Sinibaldi V, Donehower R, Leahy TL, Carr RA, Isaacson JD, Janus TJ, Andre A, Hosmane BS, Padley RJ: Atrasentan, an endothelin-receptor antagonist for refractory adenocarcinomas: safety and pharmacokinetics. J Clin Oncol 2002, 15:2171-2180 [DOI] [PubMed] [Google Scholar]
- 26.Rosanò L, Spinella F, Salani D, Di Castro V, Venuti A, Nicotra MR, Natali PG, Bagnato A: Therapeutic targeting of the endothelin A receptor in human ovarian carcinoma. Cancer Res 2003, 63:2447-2453 [PubMed] [Google Scholar]
- 27.Nelson J, Bagnato A, Battistini B, Nisen P: The endothelin axis: emerging role in cancer. Nat Rev Cancer 2003, 3:110-116 [DOI] [PubMed] [Google Scholar]
- 28.Albini A, Florio T, Giunciuglio D, Masiello L, Carlone S, Corsaro Thellung S, Cai T, Noonan DM, Schettini G: Somastatin controls Kaposi’s sarcoma tumor growth through inhibition of angiogenesis. EMBO J 1999, 13:647-655 [DOI] [PubMed] [Google Scholar]
- 29.Albini A, Iwamato Y, Kleinman HK, Martin GW, Aaronson SA, Korlowski JM, McEwan RN: A rapid in vitro assay for quantitating the invasive potential of tumor cells. Cancer Res 1987, 47:3239-3245 [PubMed] [Google Scholar]
- 30.Yu AE, Hewitt RE, Kleiner DE, Stetler-Stevenson WG: Molecular regulation of cellular invasion-role of gelatinase A and TIMP-2. Biochem Cell Biol 1997, 74:823-831 [DOI] [PubMed] [Google Scholar]
- 31.Ilic D, Damsky CH, Yamamoto T: Focal adhesion kinase: at the crossroads of signal transduction. J Cell Sci 1997, 110:401-407 [DOI] [PubMed] [Google Scholar]
- 32.Ensoli B, Gendelman R, Markham P, Fiorelli V, Colombini S, Raffeld M, Cafaro A, Chang HK, Brady JN, Gallo RC: Synergy between basic fibroblast growth factor and human immunodeficiency virus type-1 Tat protein in induction of Kaposi’s sarcoma. Nature 1994, 371:674-680 [DOI] [PubMed] [Google Scholar]
- 33.Nakahara H, Howard L, Thompson EW, Sato H, Seiki M, Yeh Y, Chen WT: Transmembrane/cytoplasmic domain-mediated membrane type 1-matrix metalloprotease docking to invadopodia is required for cell invasion. Proc Natl Acad Sci USA 1997, 22:7959-7964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Itoh Y, Takamura A, Ito N, Maru Y, Sato H, Suenaga N, Aoki T, Seiki M: Homophilic complex formation of MT1-MMP facilitates proMMP-2 activation on the cell surface and promotes tumor cell invasion. EMBO J 2001, 20:4782-4793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen WT, Wang JY: Specialized surface protrusions of invasive cells, invadopodia and lamellipodia, have differential MT1-MMP, MMP-2, and TIMP-2 localization. Ann NY Acad Sci 1999, 878:361-371 [DOI] [PubMed] [Google Scholar]
- 36.Liotta LA, Kohn EC: The microenvironment of the tumor-host interface. Nature 2001, 411:375-379 [DOI] [PubMed] [Google Scholar]
- 37.Carducci MA, Padley RJ, Breul J, Vogelzang NJ, Zonnenberg BA, Daliani DD, Schulman CC, Nabulsi AA, Humerickhouse RA, Weinberg MA, Schmitt JL, Nelson JB: Effect of endothelin-A receptor blockade with atrasentan on tumor progression in men with hormone-refractory prostate cancer: a randomized, phase II, placebo-controlled trial. J Clin Oncol 2003, 15:679-689 [DOI] [PubMed] [Google Scholar]