Figure 3. The Expression of Estrogen Receptor Protein and Signaling Proteins p-Raf, p-MEK1/2, p-MAPK, p-p90RSK, and p-Elk in LTLT-Ca Cells Compared with the Parental MCF-7Ca Cells.
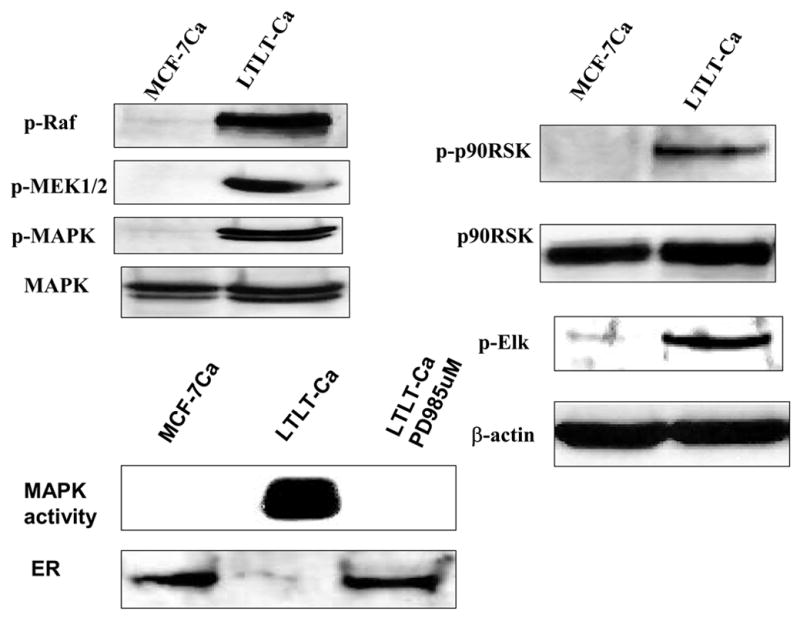
Cell lysates were prepared as described in Methods. Equal amounts of protein (60 Ag) were separated on a denaturating polyacrylamide gel and transferred to a nitrocellulose membrane. After blocking nonspecific binding with 5% nonfat milk in PBS-T, the membranes were incubated with the respective primary antibodies, and specific binding was visualized by using species-specific immunoglobulin G followed by ECL detection (ECL kit) and exposure to ECL X-ray film. After exposure to X-ray film, the membranes were stripped and probed for h-actin to confirm that equal amount of proteins were loaded in each lane. Numbers below the blots represent fold change in protein expression compared with the control obtained by densitometric analysis. For MAPK activity, assay cell lysates were prepared as described in Methods. Proteins (200 Ag) were subjected to immunoprecipitation using specific MAPK antibody. Next day, after adding kinase reaction buffer and GST fusion protein of Elk-1 (MAPK substrate), samples were incubated at 30°C for 30 minutes. Samples were analyzed by Western blotting using phospho-Elk-1 antibody. (From Jelovac et al., Cancer Res., 2005).
