Figure 2.
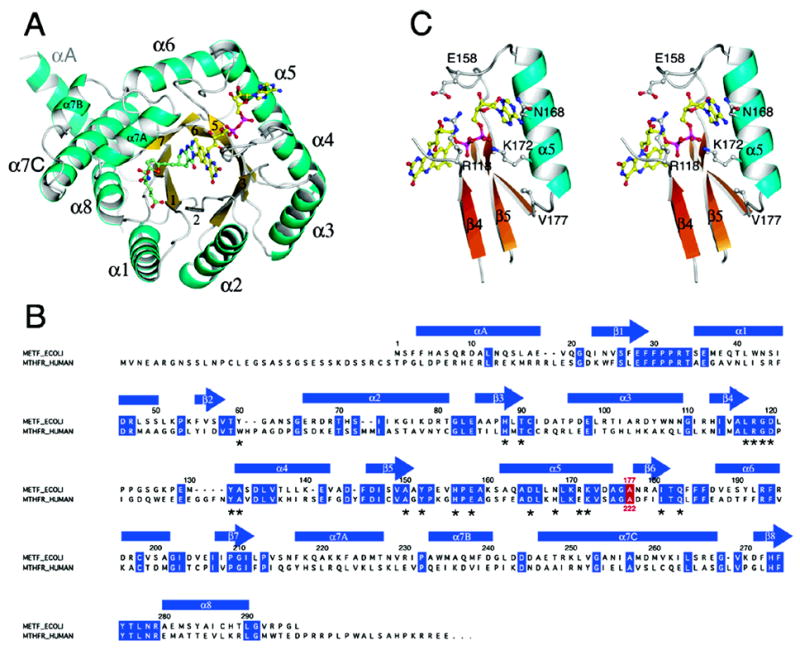
(A) The monomer of MTHFR with FAD and LY309887 shown in ball-and-stick mode. The view is along the axis of the (βα)8 barrel. Loops at the C-termini of barrel strands are referenced to the preceding strand, e.g. L4 connects β4 to α4. The groove that accommodates the folate analogue is formed by the shortened β8 strand and L8, and lies next to helix α7a. (B) Alignment of the sequence of E. coli MTHFR with the N-terminal region of human MTHFR. Secondary structures in the bacterial enzyme are displayed above the sequences, and residues that are conserved in the bacterial and human sequences are highlighted in blue. The location of Ala177/Ala222 is marked in red. Residues that interact with the FAD cofactor are marked by asterisks. (C) A stereoview of features of the binding site for FAD in the Ala177Val mutant showing Val177, helix α5, and some of the residues that interact with FAD.
