Figure 3.
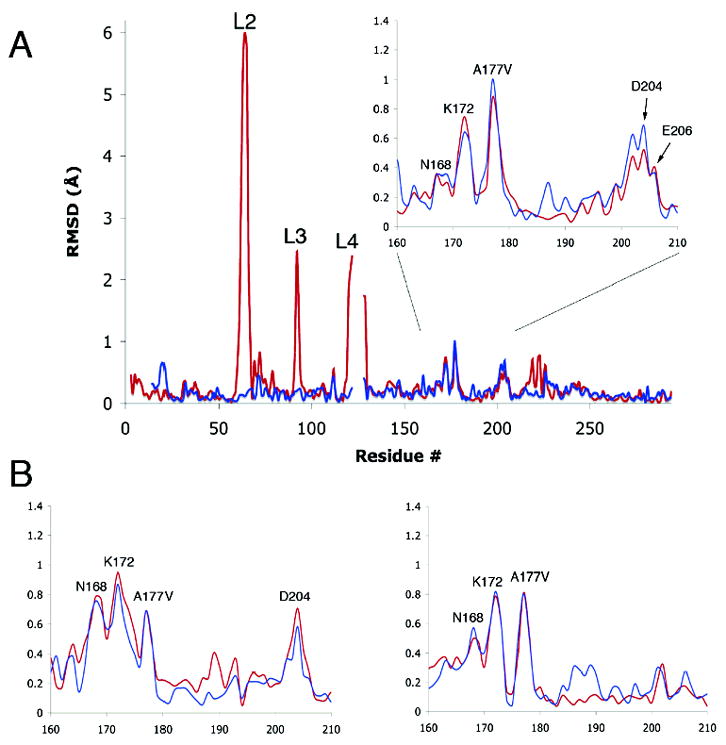
(A) RMS differences in positions of backbone Cα atoms in the B chains of wild type MTHFR (pH 7.4) and the A177V mutant, with or without bound LY309887 (pH 6.5). Differences between A177V·LY309887 and wild type are shown in red, and differences between A177V and wild type are in blue. The Ala177Val mutation primarily affects residues 165–180. Large differences are observed in loops L2, L3, and L4, which undergo pH- and ligand-induced conformational changes that are not caused by the substitution at position 177; smaller differences near 223 and 243 are attributed to folate binding. Chains were aligned as described in Materials and Methods. The inset magnifies the region in which changes are ascribed to the A → V mutation. (B) Inset regions showing the RMS difference values for the A (left) and C (right) chains.
