Figure 6.
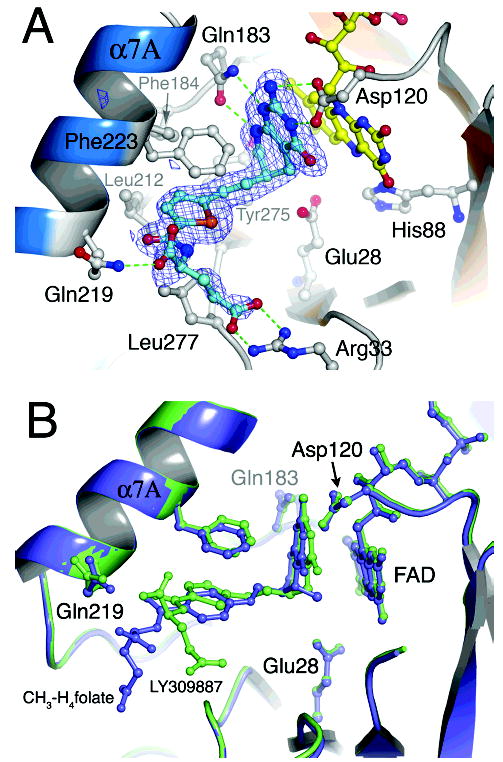
(A) A larger perspective of interactions made by the 5,10-dideazafolate, LY309887. Omit difference density, calculated after simulated annealing, is overlaid onto the final model for LY309887. The 5-deazapterin ring is oriented by hydrogen bonding interactions with Asp120 and Gln183, which are conserved among both bacterial and mammalian MTHFRs. The isoalloxazine and pterin planes are in van der Waals contact. The thiophene is sandwiched between Phe223 and Leu277, and hydrogen bonds from Gln219 and Arg33 position the α- and γ-carboxylates of the glutamate tail of deazafolate. (B) Comparison of bound LY309887 with bound methyltetrahydrofolate. The LY309887 complex of the A177V mutant (lime) is superposed on the structure of CH3-H4-folate bound to the Glu28Gln mutant of E. coli MTHFR (purple) (25). The binding modes of LY309887 and CH3-H4folate are very similar: the deazapterin of LY309887 and the pterin of CH3-H4- folate are oriented by similar interactions with Asp120 and Gln183, and the thiophene of LY309887 occupies the same hydrophobic pocket as the PABA ring (25).
