Table 2.
Oppenauer Oxidation/Brook Rearrangement/Aldolization Reactionsa
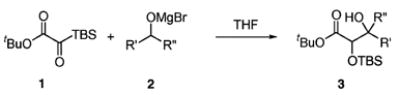
| entry | alcohol | product | yield (%)b | d.r.c |
|---|---|---|---|---|
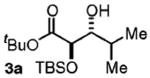
| 1d | Me2CHCH2OH |
|
97 | 10:1 |
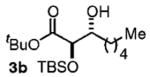
| 2d | Me(CH2)5OH |
|
86 | 7:1 |
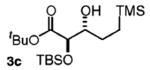
| 3d | TMS(CH2)3OH |
|
88 | 5:1 |
| 4 | CH2=CH(CH2)4OH |
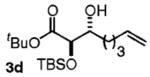
|
63 | 5:1 |
| 5 | PhCH2OH |
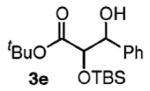
|
90 | 1.2:1 |
| 6 | 4-ClPhCH2OH |
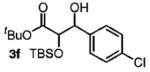
|
82 | 1:1 |
| 7 | 4-MeOPhCH2OH |
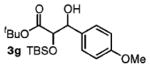
|
85 | 1:1 |
| 8 | PhCH(OH)Me |
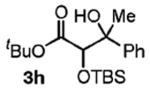
|
67 | 2.5:1 |
| 9 | cyclohexanon |
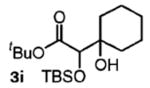
|
68 | n.a. |
Alcohol (1.5 equiv), EtMgBr (2.0 equiv), 0 °C → rt; then 1 (1.0 equiv).
Isolated yield.
Determined by 1H NMR spectroscopy; the major isomer is shown.
Reaction solvent: 2:1 THF/CH2Cl2.
