Table 1.
Optimization Summary for Asymmetric Alkene Acylationa
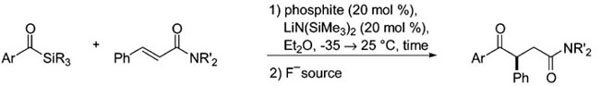
| entry | Ar | SiR3 | NR′2 | phosphite | time (h) | F− source | yield (%) | %ee |
|---|---|---|---|---|---|---|---|---|
| 1 | Ph | SiMe2Ph |
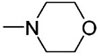
|
1-Ph | 1.0 | TBAF | 67 | 50 |
| 2 | Ph | SiMe2Ph |
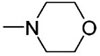
|
6b | 1.0 | TBAF | 57 | −60 |
| 3 | Ph | SiMe2Ph | NMe2 | 6b | 2.5 | TBAF | 40 | −71 |
| 4 | Ph | SiEt3 | NMe2 | 6b | 2.0 | TBAF | 44 | −88 |
| 5 | p-MeOPh | SiEt3 | NMe2 | 6b | 0.75 | TBAF | 85 | −90 |
| 6 | p-MeOPh | SiCyMe2 | NMe2 | 6b | 0.25 | TBAF | 78 | −87 |
| 7 | p-MeOPh | SiCyMe2 | NMe2 | 1-Ph | 1.0 | TBAF | 54 | 81 |
| 8 | p-MeOPh | SiCyMe2 | NMe2 | 6b | 0.25 | HF·pyr | 73 | −89 |
| 9 | p-MeOPh | SiCyMe2 | NMe2 | 6a | 1.0 | HF·pyr | 82 | 88 |
ArC(O)SiR3 (1.0 equiv), PhCH=CHC(O)NR′2 (1.5 equiv), phosphite (0.2 equiv.), and LiN(SiMe3)2 (0.2 equiv) in Et2O from −35 → 25 °C for 0.25–2.0 h after slow addition of alkene and acyl silane.
