Abstract
Circulating 25-hydroxyvitamin D [25(OH)D] is generally considered the means by which we define nutritional vitamin D status. There is much debate, however, with respect to what a healthy minimum level of circulation 25(OH)D should be. Recent data using various biomarkers such as intact parathyroid hormone (PTH), intestinal calcium absorption, and skeletal density measurements suggest this minimum level to be 80 nmol (32 ng/mL). Surprisingly, the relationship between circulating vitamin D3 and its metabolic product—25(OH)D3 has not been studied. We investigated this relationship in two separate populations: the first, individuals from Hawaii who received significant sun exposure; the second, subjects from a lactation study who received up to 6,400 IU vitamin D3/day for six months.
Results: 1) The relationship between circulating vitamin D3 and 25(OH)D in both groups was not linear, but appeared saturable and controlled; 2) Optimal nutritional vitamin D status appeared to occur when molar ratios of circulating vitamin D3 and 25(OH)D exceeded 0.3; at this point, the Vmax of the 25-hydroxylase appeared to be achieved. This was achieved when circulating 25(OH)D exceeded 100 nmol.
We hypothesize that as humans live today, the 25-hydroxylase operates well below its Vmax because of chronic substrate deficiency, namely vitamin D3. When humans are sun (or dietary) replete, the vitamin D endocrine system will function in a fashion as do these other steroid synthetic pathways, not limited by substrate. Thus, the relationship between circulating vitamin D and 25(OH)D may represent what “normal” vitamin D status should be.
Keywords: vitamin D, 25-hydroxyvitamin D, nutritional vitamin D status
Introduction
What is a normal circulating level of 25(OH)D that is sufficient to meet all physiological needs, not simply skeletal requirements in humans? In the past, this was addressed by simply sampling a diverse population of subjects who were asymptomatic for disease, measuring circulating 25(OH)D, and plotting the data using a Gaussian distribution. This approach yields normative data that are used to assess circulating 25(OH)D in that population. This is how Haddad and Chyu (1) performed their assessment of 25(OH)D status more than thirty-five years ago. They referred to their normal, asymptomatic volunteers as the normal population for circulating 25(OH)D levels. Their study also presented a group of lifeguards that had circulating 25(OH)D levels 2.5 times that of the “normals.” Countless similar studies have been performed during the ensuing decades, reiterating the same conclusion. We, however, interpret the original Haddad data differently: we suggest that the 25(OH)D levels in the sun-replete lifeguards are normal and the “normals” actually exhibit varying degrees of vitamin D deficiency.
How nutritional vitamin D deficiency is defined is a key to developing a coherent supplement policy that meets the needs of all humans. Recently, inadequate circulating 25(OH)D levels have been linked to biomarkers, including skeletal density (2–4), intestinal calcium absorption (5), secondary hyperparathyroidism (6–10), insulin secretion (11, 12), and innate immune response (13). These markers all are useful in identifying nutritional vitamin D deficiency; however, the link between 25(OH)D and vitamin D—when available at adequate concentration, remains unknown and could prove to be another important piece in understanding vitamin D metabolism. We sought to investigate this question of how 25(OH)D would respond if adequate substrate, namely vitamin D3, was always present. Thus, for this project, we studied two groups of subjects, one from a sun-rich environment and the other from a high-dose vitamin D3 supplementation study, the results of which are presented here.
Materials and Methods
Part 1. Study of Sun-Exposed Subjects
Approval for this study was granted by the University of Wisconsin Health Sciences Institutional Review Board for Human Subjects and the Committee on Human Studies at the University of Hawaii at Manoa. All subjects provided written informed consent prior to the conduct of any study procedure.
Subjects
Skin surface exposed to the sun in these subjects varied from almost total in surfers to head, arms and hands in skateboarders. Ninety-three subjects (63 males/30 females) participated in the study.
Entrance Criteria
Subjects from a sun-rich climate were recruited from the University of Hawaii at Manoa, and from patrons of the A’ala Park Board shop, Honolulu, Hawaii (latitude 21°N), in late March 2005. In order to participate, volunteers had to have met the entrance criteria of the following: a self-reported sun exposure time of three or more hours per day on five or more days per week for at least the preceding three months. Those who met entrance criteria were enrolled in the study following written informed consent.
Study Protocol
Blood was collected for serum 25(OH)D and vitamin D3 measurement when they were interviewed.
All participants completed a non-validated, self-administered questionnaire, which included questions about sun exposure, sunscreen use, and dietary vitamin D intake.
To document sun exposure, skin color was measured by reflectance colorimetry (IMS SmartProbe, Millford, CT). A measurement was taken on the back of the hand and the front of the distal thigh for the darkest measurement and under the arm and at the self-reported least sun-exposed area—often the breast or buttock, to determine the lightest or natural skin color. A previously developed sun exposure index (14), which utilizes the rule of nines was used to estimate the amount and duration of skin sun exposure.
Circulating 25(OH)D was measured on all 93 subjects using an RIA as previously reported (15).
Data Analysis: The 10 highest and 10 lowest circulating 25(OH)D levels were selected to determine circulating vitamin D3 levels using direct ultraviolet (UV) detection following high performance liquid chromatography as previously described (16). The data were plotted using a best-fit regression analysis with vitamin D3 serving as the independent variable.
Part II. Study of High Dose Vitamin D3 Supplemented Subjects
Approval for this study was granted by the Medical University of South Carolina’s (MUSC) Institutional Review Board for Human Subjects, HR #11345 and the General Clinical Research Center (GCRC; Protocol #694). Fully lactating mothers (17) within one month postpartum were eligible for inclusion in the study if they planned to continue full breastfeeding for the next six months. The subjects were randomly divided into two groups. Exclusion criteria included preexisting type I or type II diabetes, hypertension, parathyroid disease, and uncontrolled thyroid disease. Subjects were compensated for their participation with gift cards given at the end of each visit.
Study Design
Women in the high dose supplementation group of a larger randomized, double-blind, placebo-control trial were included in this study. Following written informed consent, mothers were randomized to one of two vitamin D supplementation regimens: Group 1: 400 IU vitamin D3/day (0 IU vitamin D3—placebo and 1 prenatal vitamin containing 400 IU vitamin D3), or Group 2: 6,400 IU vitamin D3/day (6,000 IU vitamin D3 and 1 prenatal vitamin containing 400 IU vitamin D3).
Study Protocol
Following written informed consent, each mother had baseline serum samples collected for measurement of circulating 25(OH)D and vitamin D3.
The mothers were started on a total of 400 or 6,400 IU vitamin D3 tablets/day (Tishcon Corporation, Westberry, NY, a Good-Manufacturing-Practice (GMP) facility that met FDA production guidelines) for up to 6 months.
Serum samples were collected at monthly intervals and analyzed for circulating 25(OH)D and vitamin D3 as described for the sun-rich population in Part I (Methods).
Data Analysis: The data were plotted as in Part I of the study using a best-fit regression analysis with vitamin D3 serving as the independent variable.
Results
Subjects from the sun-rich environment exhibited a wide range of circulating 25(OH)D levels (11–71 ng/mL). Similarly, the range of circulating 25(OH)D levels in women in the supplementation group was from 12–77 ng/mL. This wide range also was observed for the circulating vitamin D3 levels (<1–64 ng/mL in the sun-rich environment group and <1–75 in the supplementation group). When data from the 20 subjects in the lowest and highest quartiles, comparing circulating 25(OH)D and vitamin D3 were plotted, a significant second-order equation was generated (p<0.0001, Figure 1). Similarly, when the data from the supplementation group was plotted, a similar, significant second-order equation was observed (p<0.0001, Figure 2). These equations did not differ statistically (p>0.15).
Figure 1. Circulating 25(OH)D as a Function of Vitamin D3 Status in Subjects from a Sun-Rich Environment.
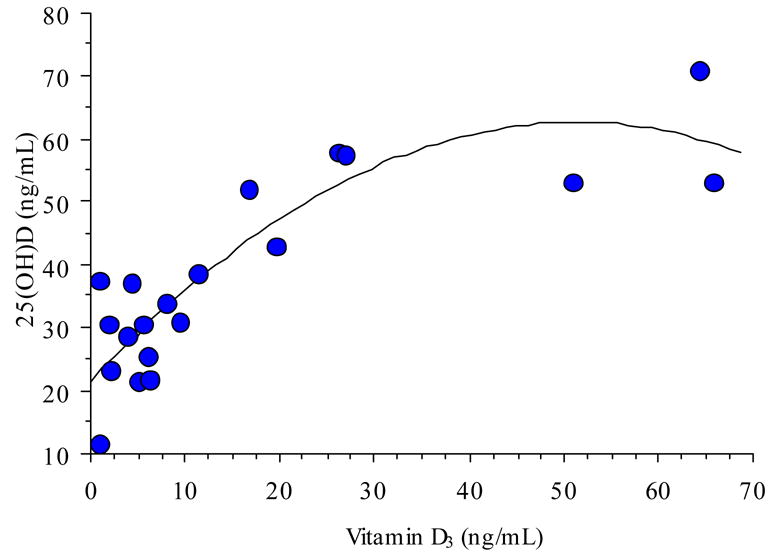
Circulating 25(OH)D as a function of circulating vitamin D3 in a sun-exposed population. Best-fit regression analysis yielded the equation y=21.5 + 1.6X − 0.02X2, r2=0.75, n=20.
Figure 2. Circulating 25(OH)D as a Function of Vitamin D3 Status in Supplemented Subjects.
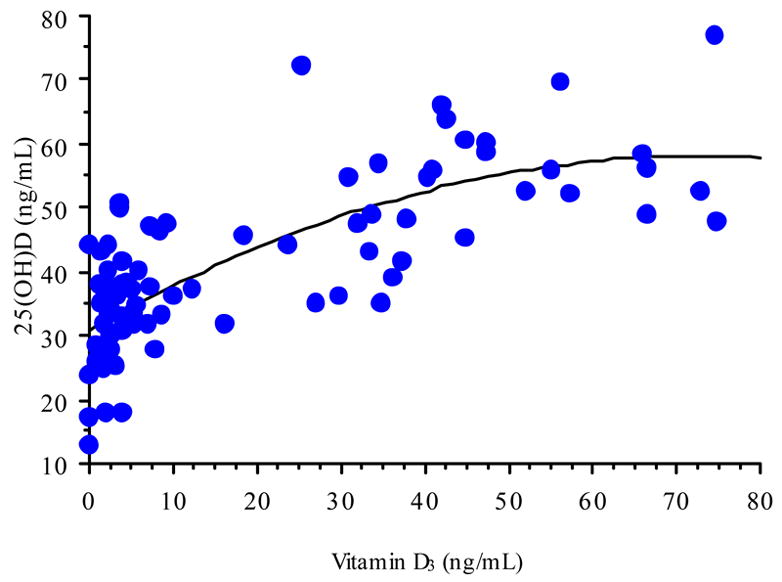
Circulating 25(OH)D as a function of circulating vitamin D3 in high dose (6,400 IU vitamin D3/day) subjects. Best-fit regression analysis yielded the equation y = 30.8 + 0.7x − 0.01X2, r2= 0.60, n=82.
Discussion
The question, what is a “normal” nutritional vitamin D status, is currently a hotly debated topic. Historically, a “normal” nutritional vitamin D status has been defined as just about any circulating level of 25(OH)D in asymptomatic subjects (1, 18). Recently, attempts have been made to reevaluate this “normal” circulating level of 25(OH)D using biomarkers such as parathyroid hormone (6–10), intestinal calcium absorption (5), skeletal density (2–4), glucose clearance (12), and innate immune function (13). Generally, these studies suggest that a minimum circulating level of 25(OH)D should be >80 nmol (32 ng/mL) (18).
In the present study, we sought to investigate what circulating 25(OH)D levels would result in populations exhibiting no substrate limitations to the vitamin D-25-hydroxylase. To perform this, we chose two distinct populations. The first were individuals from a year-found sunny environment who spent a good deal of time outdoors. The second were a group of lactating women receiving a substantial daily oral dose of vitamin D3. Surprisingly, a study such as this previously had not been undertaken. There are several reasons for this. First, finding a group of sun-exposed individuals is not an easy task; in fact, we had to go to Hawaii to find them. Secondly, very few studies have been performed where subjects actually received adequate vitamin D3 supplementation to make them replete. Finally, it is very difficult and costly to measure circulating vitamin D3 and relate it to circulating 25(OH)D. The results of our study are far-reaching.
At a maternal intake of 6,400 IU vitamin D3/day, circulating vitamin D3 increased dramatically. Maternal circulating 25(OH)D also increase; however, the increase appeared to be limited and controlled (Figure 2). A similar relationship was observed in the sun-exposed individuals (Figure 1). In these individuals, sun exposure was greater than fifteen hours/week—although not all had total body exposure, some only hands, arms, and head. The data from our study suggests the following: The relationship between circulating vitamin D3 and 25(OH)D is not linear in either case; rather it appears saturable and controlled. This suggest either/or product-substrate inhibition of the vitamin D-25-hydroxylase. Optimal nutritional vitamin D status may occur when approaching equimolar concentrations of circulating vitamin D3 and 25(OH)D (>100 nmol). At this point, the Vmax of the enzyme appears to be achieved. It is important to note that as humans live today, the vitamin D-25-hydroxylase operates well below its Vmax because of chronic substrate (vitamin D) deficiency. Not a single other steroidal hormone system in the body is limited in this fashion since their starting point is cholesterol. When humans are sun- (or dietary-) replete, the vitamin D endocrine system will function in a fashion as do these other steroid synthetic pathways, not limited by substrate availability.
This study also demonstrates that individuals can be vitamin D deficient with significant sun exposure if the skin area exposed is limited as was suggested several years ago (19). Finally, whether one receives their vitamin D3 orally or through UV exposure, the vitamin D-25-hydroxylase appears to handle it in an equivalent fashion with respect to maintaining circulating 25(OH)D levels. Thus, we believe that the relationship between circulating vitamin D and 25(OH)D may define adequate nutritional vitamin D status.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Haddad JG, Chyu K. Competitive protein-binding radioassay for 25-hydroxycholecalciferol. J Clin Endocrinal Metab. 1971;33:992–995. doi: 10.1210/jcem-33-6-992. [DOI] [PubMed] [Google Scholar]
- 2.Bischoff-Ferrari H, Dietrich T, Orav E, Dawson-Hughes B. Positive association between 25(OH)D levels and bone mineral density: A population-based study of younger and older adults. Amer J Med. 2004;116:634–639. doi: 10.1016/j.amjmed.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 3.Fuleihan E, Nabulsi M, Tamim H, Maalouf J, Salamoun M, Khalife H, et al. Effect of vitamin D replacement on musculoskeletal parameters in school children: A randomized controlled trial. J Clin Endocrinal Metab. 2006;91:405–412. doi: 10.1210/jc.2005-1436. [DOI] [PubMed] [Google Scholar]
- 4.Javaid M, Crozier S, Harvey N, Gale C, Dennison E, Boucher B, et al. Maternal vitamin D status during pregancy and childhood bone mass at 9 years: A longitudinal study. Lancet. 2006;367:36–43. doi: 10.1016/S0140-6736(06)67922-1. [DOI] [PubMed] [Google Scholar]
- 5.Heaney R, Dowell M, Hale C, Bendich A. Calcium absorption varies within the reference range for serum 25-hydroxyvitamin D. J Amer College Nutr. 2003;22(2):142–146. doi: 10.1080/07315724.2003.10719287. [DOI] [PubMed] [Google Scholar]
- 6.Lips P, Wiersinga A, Van Ginkel FC, Jongen MJ, Netelenbos JC, Hackeng WH, et al. The effect of vitamin D supplementation on vitamin D status and parathyroid function in elderly subjects. J Clin Endocrinol Metab. 1988;67:644–650. doi: 10.1210/jcem-67-4-644. [DOI] [PubMed] [Google Scholar]
- 7.Gloth FM, Gundberg CM, Holllis BW, Haddad JG, Tobin JD. Vitamin D deficiency in homebound elderly persons. JAMA. 1995;274:1683–1686. doi: 10.1001/jama.1995.03530210037027. [DOI] [PubMed] [Google Scholar]
- 8.Visser M, Deeg D, Lips P. Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): The longitudinal aging study Amsterdam. J Clin Endocrinal Metab. 2003;88:5766–5772. doi: 10.1210/jc.2003-030604. [DOI] [PubMed] [Google Scholar]
- 9.Vieth R, Ladak Y, Walfish P. Age-related changes in the 25-hydroxyvitamin D versus parathyroid hormone relationship suggest a different reason why older adults require more vitamin D. J Clin Endocrinal Metab. 2003;88:185–191. doi: 10.1210/jc.2002-021064. [DOI] [PubMed] [Google Scholar]
- 10.Chel VG, Ooms ME, Popp-Snijders C, Pavel S, Schothorst AA, Meulemans CCE, et al. Ultraviolet irradiation corrects vitamin D deficiency and suppresses secondary hyperparathyroidism in the elderly. J Bone Mineral Res. 1998;13:1238–1242. doi: 10.1359/jbmr.1998.13.8.1238. [DOI] [PubMed] [Google Scholar]
- 11.Lee S, Clark S, Gill R, Christakos S. 1,25-Dihydroxyvitamin D3 and pancreatic beta-cell function: vitamin D receptors, gene expression, and insulin secretion. Endocrinology. 1994;134(4):1602–1610. doi: 10.1210/endo.134.4.8137721. [DOI] [PubMed] [Google Scholar]
- 12.Chiu K, Chu A, Go V, Soad M. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Amer J Clin Nutr. 2004;79:820–825. doi: 10.1093/ajcn/79.5.820. [DOI] [PubMed] [Google Scholar]
- 13.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik S, et al. Toll-Like Receptor Triggering of a Vitamin D-Mediated Human Antimicrobial Response. Science. 2006:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 14.Barger-Lux MJ, Heaney RP. Effects of Above Average Summer Sun Exposure on Serum 25-Hydroxyvitamin D and Calcium Absorption. J Clin Endocrinol Metab. 2002;87(11):4952–4956. doi: 10.1210/jc2002-020636. [DOI] [PubMed] [Google Scholar]
- 15.Hollis BW, Kamerud JQ, Selvaag SR, Lorenz JD. Determination of vitamin D status by radioimmunoassay with a 125I-labeled tracer. Clin Chem. 1993;39:529–533. [PubMed] [Google Scholar]
- 16.Hollis BW. Detection of vitamin D and its major metabolites. In: Feldman D, Glorieux F, Pike J, editors. Vitamin D. New York, N.Y.: Academic Press; 2005. pp. 932–950. [Google Scholar]
- 17.Coffin CF, Labbok MH, Belsey M. Breastfeeding definitions. Contraception. 1997;55:323–325. doi: 10.1016/s0010-7824(97)00039-5. [DOI] [PubMed] [Google Scholar]
- 18.Hollis BW. Circulating 25-hydroxyvitamin D levels indicative of vitamin sufficiency: Implications for establishing a new effective DRI for vitamin D. J Nutr. 2005;135:317–322. doi: 10.1093/jn/135.2.317. [DOI] [PubMed] [Google Scholar]
- 19.Matsuoka LY, Wortsman J, Hollis BW. Use of topical sunscreen for the evaluation of regional synthesis of vitamin D3. J Amer Acad Dermatol. 1990;22:772–775. doi: 10.1016/0190-9622(90)70107-s. [DOI] [PubMed] [Google Scholar]


