Figure2.
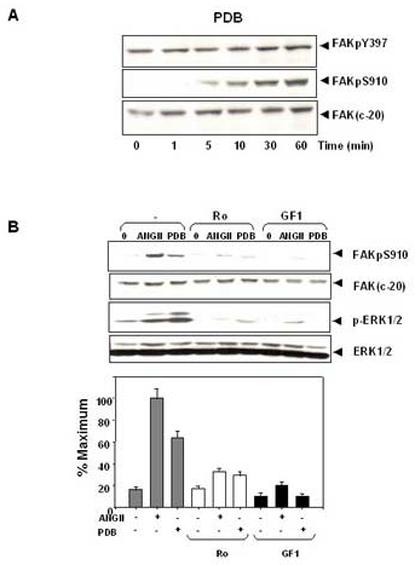
ANG II induces FAK phosphorylation at Ser-910 through a PKC-dependent pathway. (A). Time course of FAK phosphorylation at Ser-910 induced by PDB in IEC-18 cells. Confluent and quiescent cells were treated at 37° C with 100nM PDB for various times as indicated and were subsequently lysed. FAK phosphorylation at Ser-910 or Tyr-397 was analyzed by Western blotting with pSer-910 Ab or pTyr-397 Ab. The membranes were further analyzed by Western blotting using anti-FAK Ab. (B). PKC inhibitors prevent FAK phosphorylation at Ser-910 induced by either ANGII or PDB in IEC-18 cells. Upper Panel: Confluent and quiescent cells were treated for 1 h either in the absence (−) or presence of 2.5 μM Ro-31-8220 or 5μM GF-109203X at 37° C. Cells were then incubated for further 10 min either with 50 nM ANGII or 100 nM PDB. The cells were lysed and the extracts were analyzed by Western blotting with pSer-910 Ab. The membranes were further analyzed by Western blotting using anti-FAK Ab. An aliquot of the lysates was analyzed by Western blotting with phospho-p44/42 MAP Kinase Ab (pERK1/2). These membranes were further analyzed by Western blotting using anti-ERK1/ 2 Ab. Lower Panel: Quantification of the inhibition of Ser-910 phosphorylation by these two PKC inhibitors was performed by scanning densitometry. Values shown as bars are the mean of at least three independent experiments and are expressed as the percentage of the maximal increase of Ser-910 phosphorylation above control (unstimulated) values. In all cases, the autoradiograms shown are representative of at least three independent experiments.
