Figure 4.
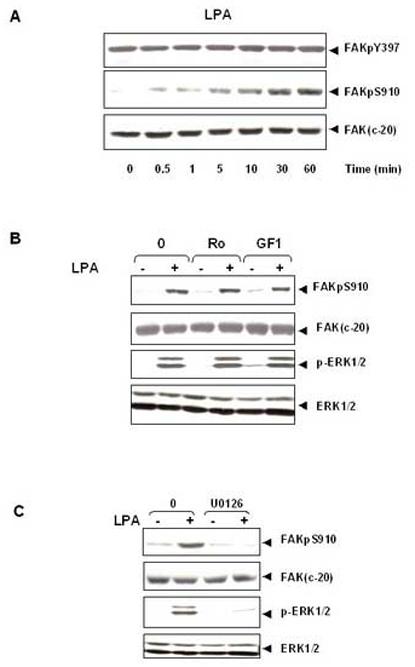
LPA induces FAK phosphorylation at Ser- 910 through PKC-independent, ERK-dependent pathway. (A). Time-course of LPA in stimulation of FAK phosphorylation at Ser-910 in IEC-18 cells. Confluent and quiescent cells were treated at 37° C with 10μM LPA for various times as indicated and were subsequently lysed. FAK phosphorylation at Ser-910 or Tyr-397 was analyzed by Western blotting with pSer-910 Ab or pTyr-397Ab. The membranes were further analyzed by Western blotting using anti-FAK Ab. The autoradiograms shown are representative of at least three independent experiments. (B).LPA stimulates FAK phosphorylation at Ser-910 independent of PKC. Confluent and quiescent cells were treated for 1 h either in the absence (−) or presence of 2.5 μM Ro-31-8220 or 5μM GF-109203X at 37° C. Cells were then incubated for further 10 min with 10μM LPA. The cells were lysed and the extracts were analyzed by Western blotting with pSer-910 Ab. The membranes were further analyzed by Western blotting using anti-FAK Ab. An aliquot of the lysates was analyzed by Western blotting with phospho-p44/42 MAP Kinase Ab (pERK1/2). These membranes were further analyzed by Western blotting using anti-ERK1/ 2 Ab. (C). The MEK inhibitor U0126 prevents LPA induced phosphorylation of FAK at Ser-910 in IEC-18 cells. Cells were incubated with 10μM U 0126 for 1 h at 37°C and then stimulated with 10μM LPA for 10 min. The cells were lysed and the extracts were analyzed by Western blotting with pSer-910 Ab. The membranes were further analyzed by Western blotting using anti-FAK Ab. A portion of the lysates was analyzed by Western blotting with pERK1/2 Ab. These membranes were further analyzed by Western blotting using ERK1/2 Ab. In all cases, the autoradiograms shown are representative of at least three independent experiments.
