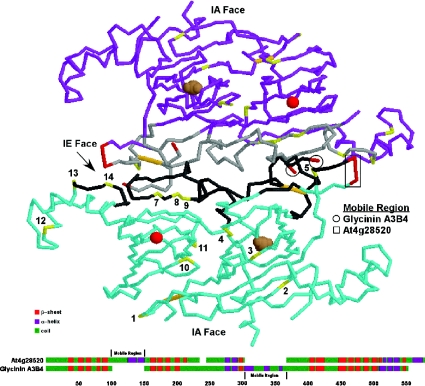
Figure 5. Superimposition of A. thaliana cruciferin phosphorylation sites on the glycinin tertiary structure.
Each glycinin and cruciferin molecule consists of an α- and β-peptide linked together by intrachain (IA) and interchain (IE) disulfide bonds (orange) that denote the two faces. Although the mature proteins form hexameric structures, for the sake of clarity only two interacting glycinin A3B4 molecules (blue and purple) are depicted. The phosphorylated residues (yellow and numbered according to Figure 1) tend to cluster along the IE face and associate with the binding regions (grey) involved in subunit interactions. Magnesium (red sphere) and carbonate (brown spheres) ions with the barrel of the cupin domains are shown. A comparison of the predicted secondary structures of the cruciferin encoded by At4g28520 and glycinin A3B4 is provided below. Each protein contains a highly charged loop (red) extending from the regions indicated, and both regions are located in the same general vicinity on the three-dimensional structure. These may serve as the mobile regions preventing non-specific interactions with the IE face prior to processing and hexamer assembly.