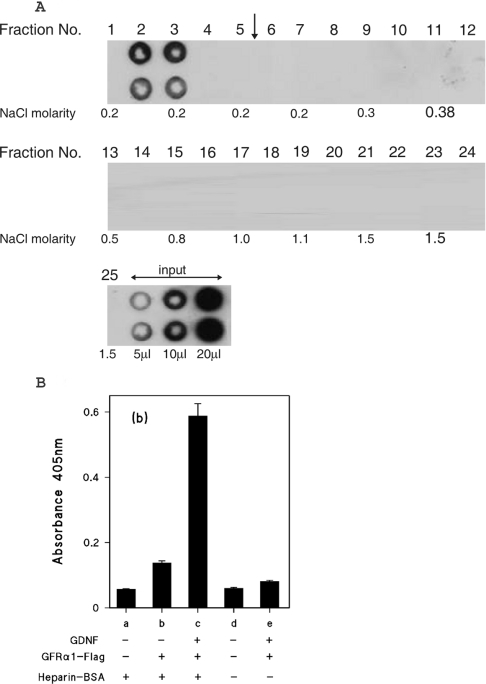
Figure 6. Heparin affinity chromatography of GFRα1–FLAG chimaeric protein.
(A) GFRα1–FLAG partially purified by ion-exchange chromatography was applied to a heparin Hi-trap column as described in the Experimental section for GDNF, except that the linear NaCl gradient was 0.18–2 M. The gradient was applied after fraction 5, as indicated by the vertical arrow. The NaCl molarities of the odd numbered fractions, as determined by conductivity measurements are shown. (B) Binding of GFRα1–FLAG to heparin-immobilized GDNF. GDNF (2.5 ng/well) was captured on wells coated with either heparin–BSA complex (columns a–c) or mock-conjugated BSA (columns d and e) as described for the heparin-binding ELISA. GFRα1–FLAG was then added to wells (columns b, c and e) at a quantity of 20 ng/well, as estimated by comparison with the GFRα1–Fc Western blotting. All wells were developed with anti-FLAG and alkaline phosphatase-labelled second antibody. The results are shown as means±S.E.M. for four replicate wells from a single representative experiment.