Figure 2.
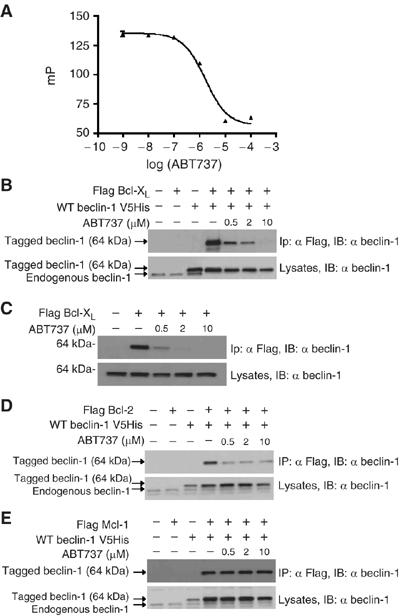
Inhibition of the interaction between Beclin-1 and anti-apoptotic Bcl-2 proteins by ABT737. (A) Competition between Beclin-1 BH3 and ABT737 for Bcl-XL binding. A fluorescent 25-mer peptide containing the BH3-like domain of Beclin-1 (Figure 1D) was docked to recombinant Bcl-XL ΔTM protein in the absence or presence of ABT737. The IC50 of ABT737, as measured in the presence of 15 nM of peptide and 100 nM of Bcl-XL ΔTM, was 1.7 μM. (B, C) Abolition of the interaction between Bcl-XL and Beclin-1 by ABT737 in intact cells. Co-immunoprecipitation assays were performed on HeLa cells transfected 48 h earlier with the indicated constructs as in Figure 1E. Sixteen hours before the immunoprecipitation, cells were exposed to ABT737. Similar results were obtained for co-transfected Bcl-XL and Beclin-1 (B) and for endogenous Beclin-1 interacting with Flag-tagged Bcl-XL (C). (D, E) Differential effect of ABT737 on the interaction between Bcl-2 (D) or Mcl-1 (E) and Beclin-1. This experiment was designed as (B). All experiments have been performed at least three times, with similar results.
