Figure 4.
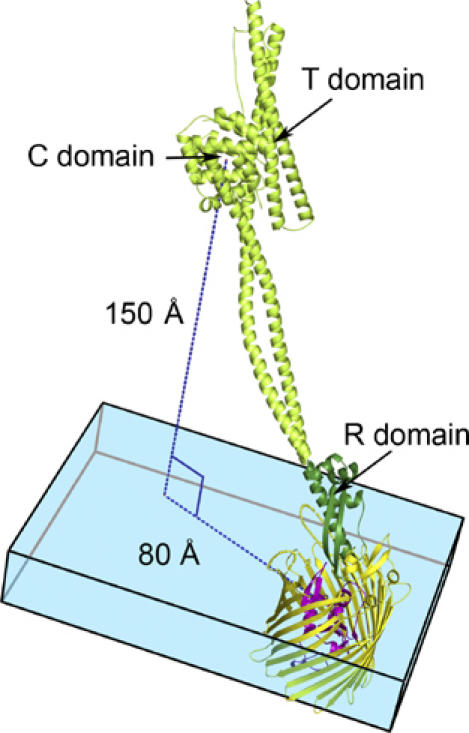
A model of full-length colicin Ia bound to Cir. Because no conformational changes occur in the R-domain of colicin Ia upon binding Cir (see text and Supplementary Figure 3), we assume that the remainder of colicin Ia does not change conformation upon binding. With this assumption, we have superimposed the R-domain of colicin Ia from the Cir–colicin complex structure with full-length colicin Ia (PDB ID 1CII). In the model, the colicin Ia R-domain is shown in dark green, with the rest of the colicin molecule (present only in the full-length colicin Ia structure) shown in light green. Cir is colored yellow and magenta, as in previous figures. The blue box denotes the thickness of the membrane bilayer, with Cir embedded in it. Colicin Ia binds to Cir with an angle of approximately 45° to the membrane bilayer, positioning the translocation (T) and channel-forming (C) domains of colicin Ia about 150 Å above the bilayer and translated by about 80 Å from Cir. Both the T- and C-domains are thought to enter the periplasm by a transport mechanism involving Cir and TonB–ExbB–ExbD.
