Figure 2.
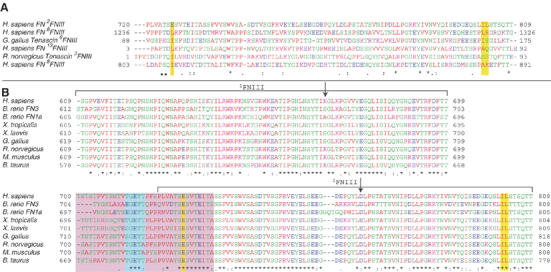
CLUSTAL-W sequence alignment of (A) 2FNIII and the five closest structural homologs of 2FNIII as identified by Dali (Holm and Sander, 1998), and (B) 1−2FNIII sequences from different FN proteins. The residue spans of 1FNIII and 2FNIII are denoted by brackets, and the positions of the two amino-acid substitutions engineered in this study, K669A and D767A, are indicated by arrows. Highlighted by yellow bars are the three 2FNIII residues (E726, I802 and L803) identified in our analysis as possibly responsible for the disordered 2FNIII N-terminus, and their sequence counterparts in other domains. Highlighted in a purple box are the residues of the disordered 1FNIII–2FNIII linker in all FN molecules. Shown in a cyan box are residues of the interdomain linker removal of which adversely affects the 1−2FNIII–FN30 kDa interaction.
