Figure 1.
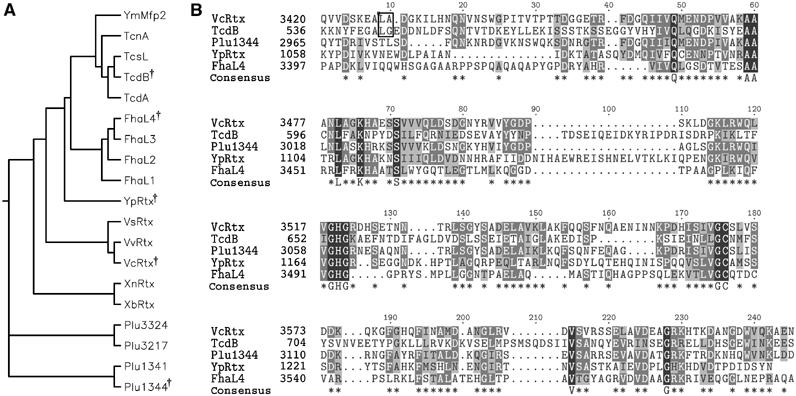
Alignment of putative cysteine protease domains. (A) Phylogenetic tree of 19 putative CPDs and (B) CLUSTALW alignment of five diverse sequences. †Symbol in phylogenetic tree indicates diverse sequences selected for alignment in (B). In (B), asterisks in consensus sequence represent conserved residues in 3/5 sequences and capital letters represent 100% identity. Outlined dipeptide sequence indicates cleavage sites determined experimentally. CPDs were identified within nine Vibrio-type RTX toxins from V. cholerae (VcRtx); V. vulnificus (VvRtx), V. splendidus (VsRtx), Xenorhabdus nematophila (XnRtx), X. bovienii (XbRtx), and Photorhabdus luminescens (Plu1344, Plu1341, Plu3217, and Plu3324); four clostridial toxins, specifically C. difficile toxin A (TcdA), toxin B (TcdB), C. sordellii cytotoxin L (TcsL), and C. noveyi alpha toxin (TcnA); two putative Yersinia toxins Y. pseudotuberculosis YPTB3219 (YpRtx) and Y. mollaretti Mfp2 (YmMfp2); and four domains arranged in tandem in B. pertussis putative adhesin FhaL (FhaL1-4).
